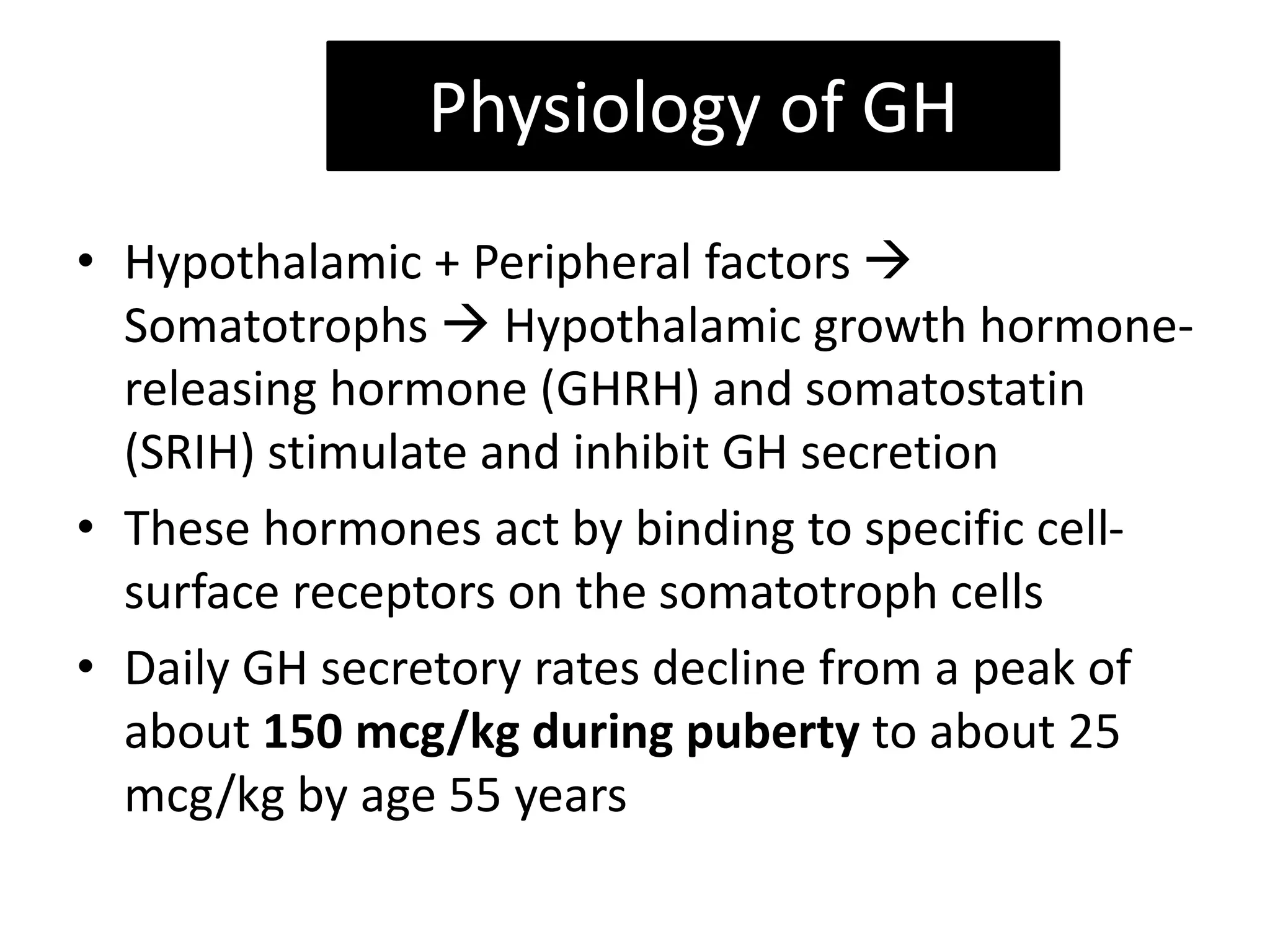
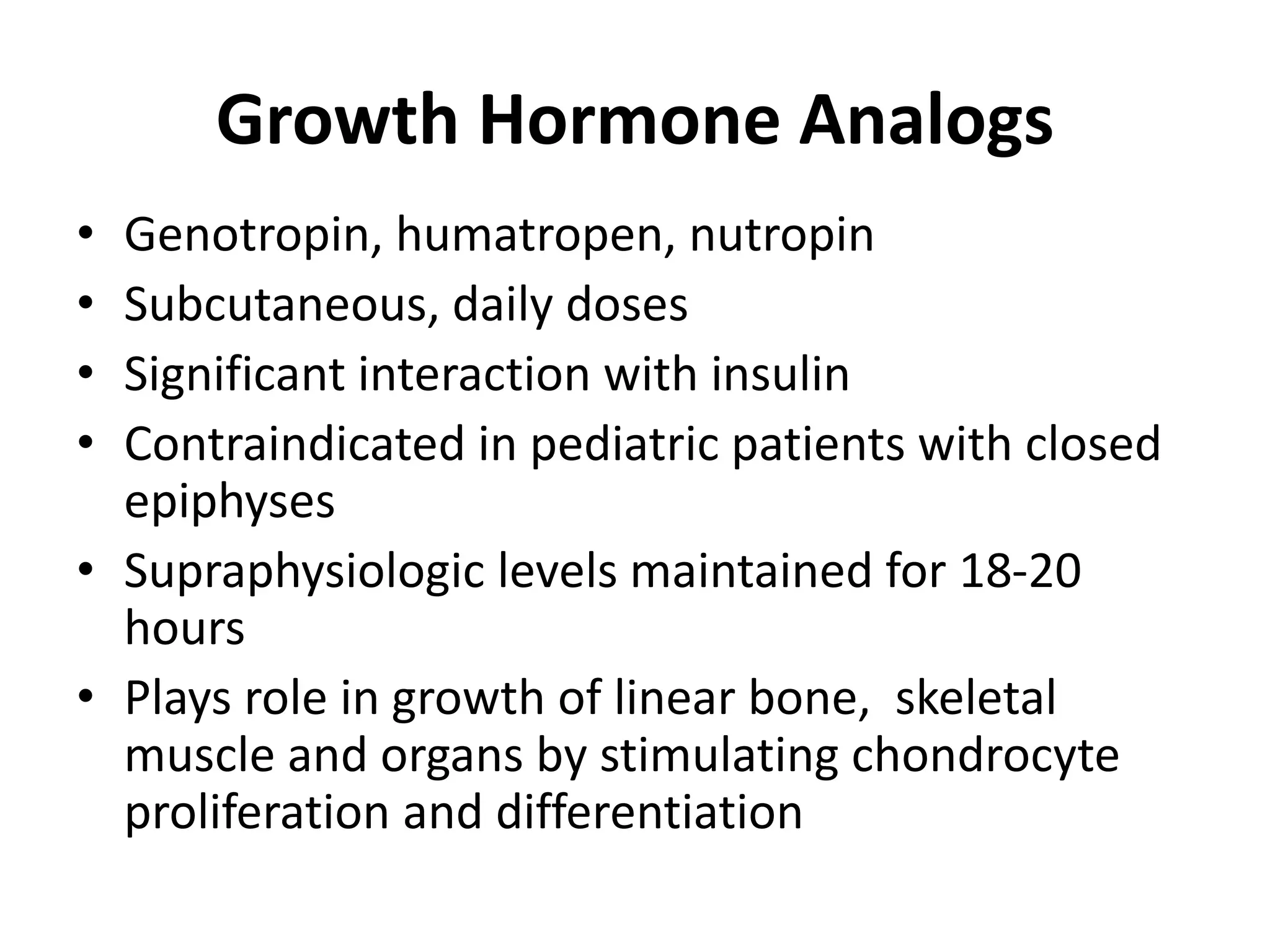
The document discusses growth hormone (GH) deficiency. It notes that GH is produced by the pituitary and regulates growth. GH secretion peaks during puberty then declines with age. Causes of GH deficiency include genetic factors, tumors, injuries and infections. Clinical features include short stature, delayed development, and body proportions differences. Evaluation involves assessing growth rates and examinations. Treatment is usually GH injections, aimed at restoring normal growth rates. Response is monitored through height velocity measurements.