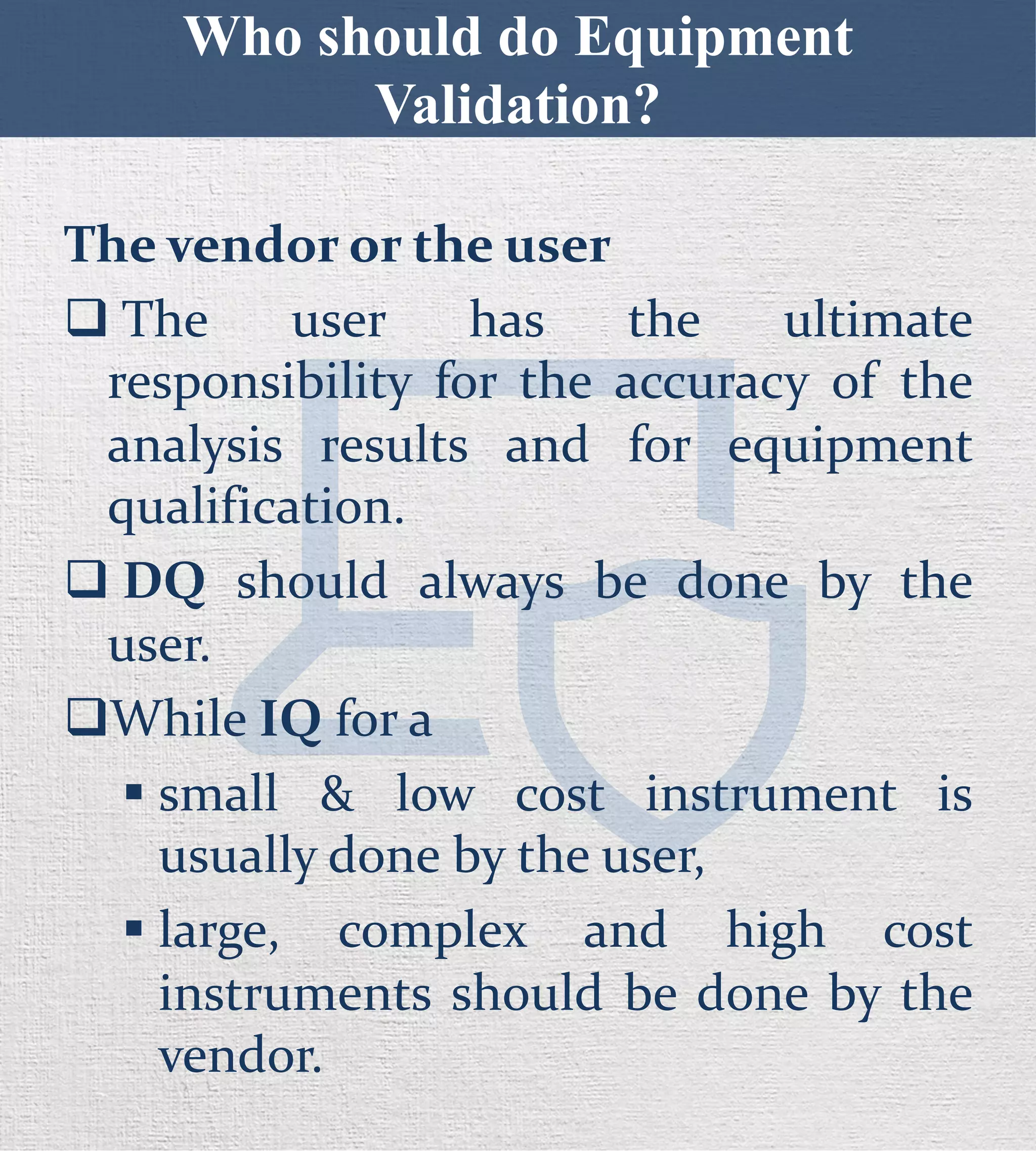
The document discusses the stages of equipment qualification which include design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). It states that DQ establishes functional and operational specifications and should be done by the user. While the vendor performs IQ for large, complex instruments, the user performs it for small, low-cost ones. OQ and PQ can be done by either the vendor or user, but PQ should always be done by the user since the applications are specific. The document provides details on the contents and objectives of each qualification stage.