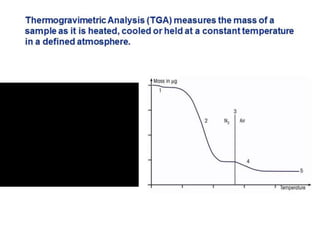
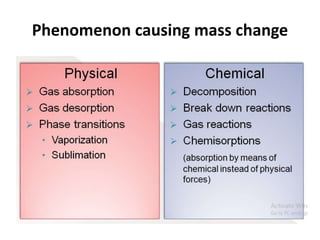
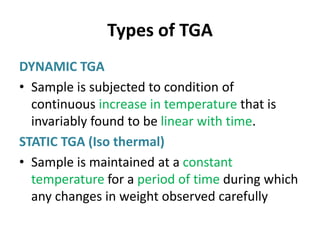
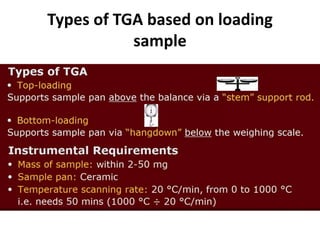
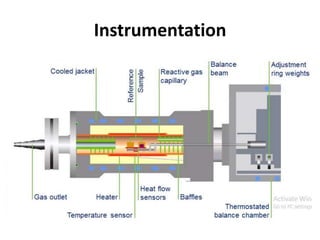
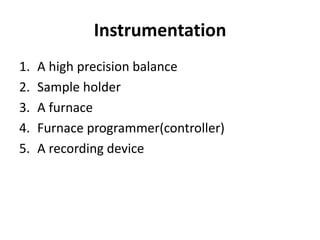
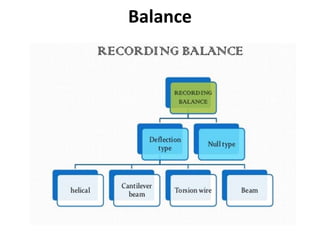
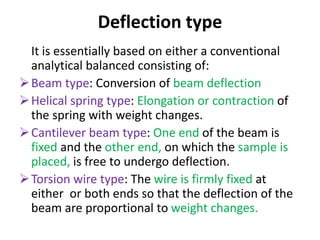
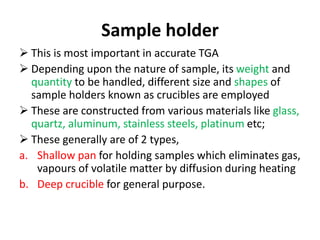
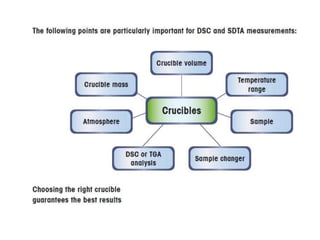
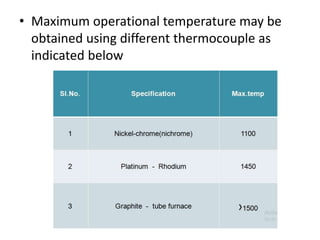
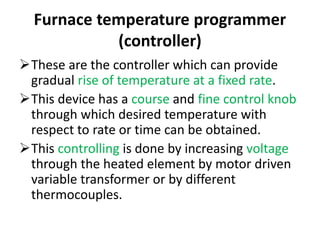
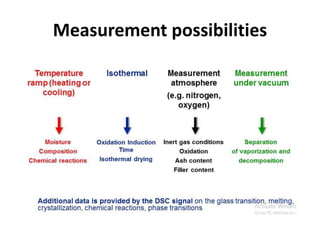
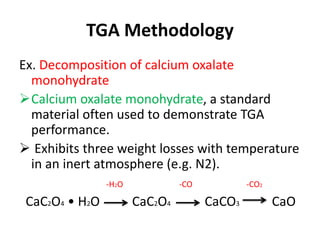
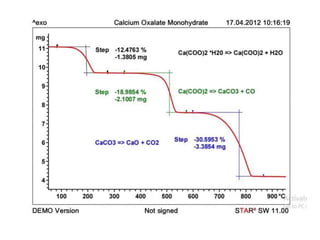
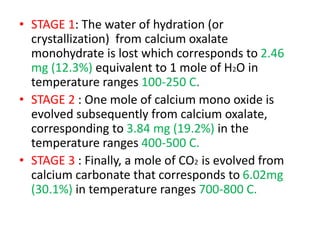
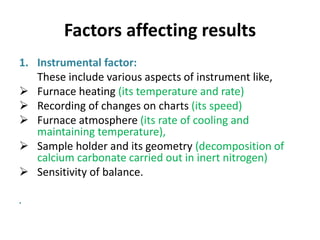
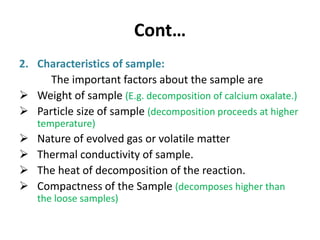
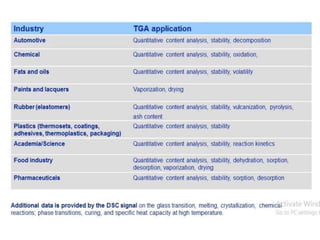
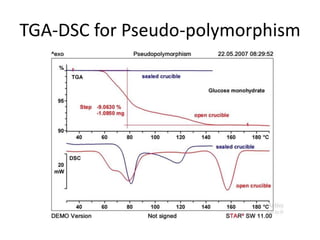
Thermal analysis techniques measure how physical properties of materials change with temperature. Thermogravimetric analysis (TGA) specifically measures changes in mass with temperature or time in a controlled atmosphere. TGA works by heating a sample and measuring its weight loss, which provides information about decomposition reactions and thermal stability. It works by slowly heating a sample in a controlled furnace under an inert gas and precisely measuring weight changes with a high-precision balance. Factors like heating rate, sample amount and particle size can affect TGA results. TGA has applications in fields like polymers, ceramics, medicines and foods for properties analysis, reaction kinetics studies and quality control.