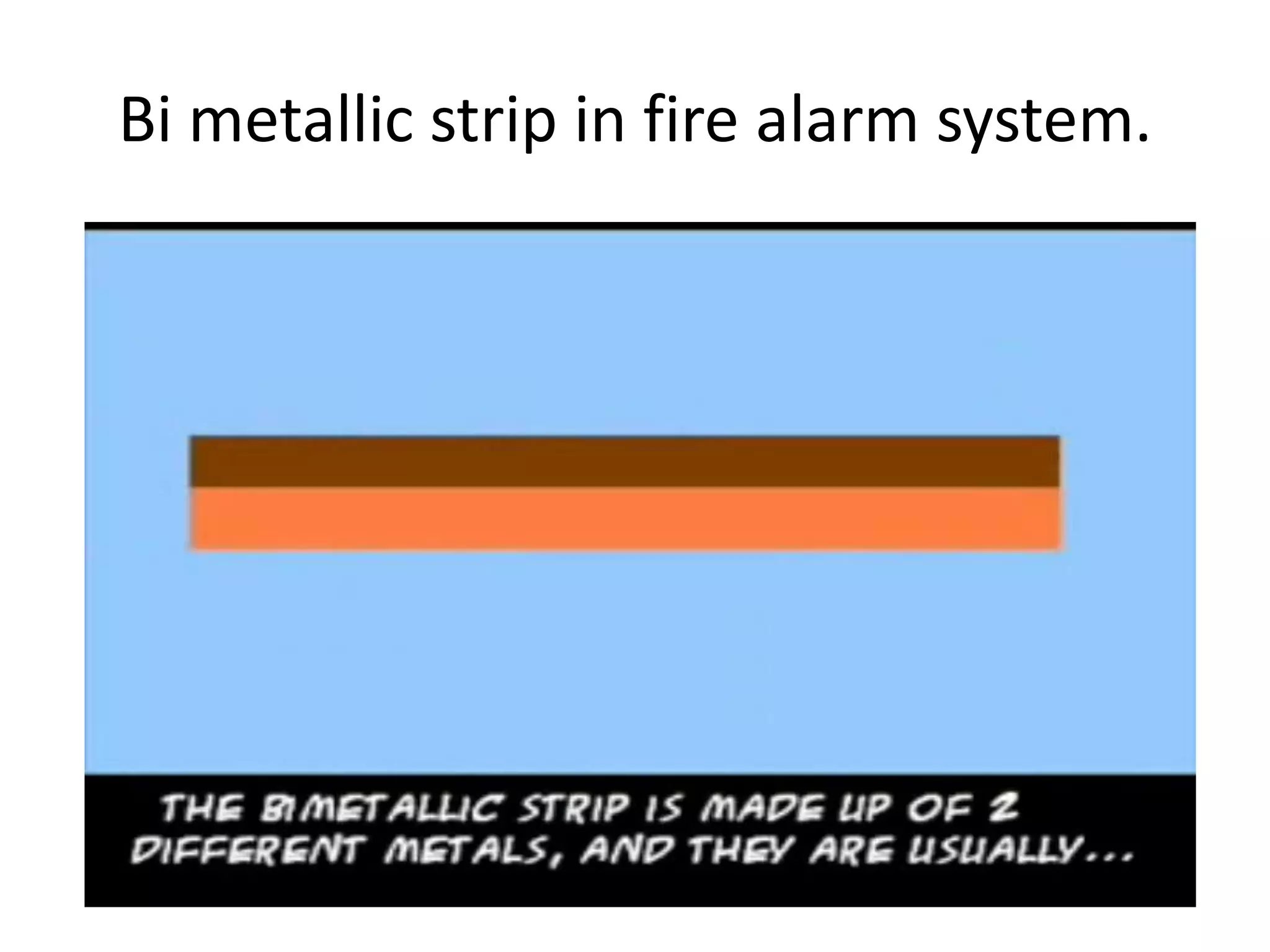
The document covers thermal physics, focusing on thermal conduction, thermal expansion, and thermodynamics, including its laws and concepts such as the Otto and Diesel cycles. It explains the principles of bimetallic strips and methods to measure thermal conduction in different materials. The content highlights key thermodynamic principles, including energy conservation and the behavior of heat engines.