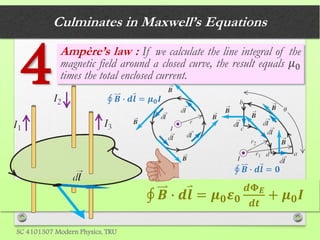
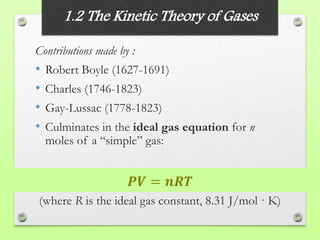
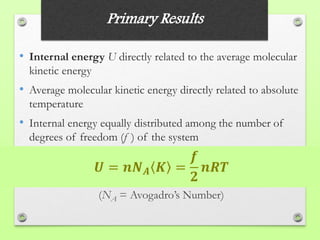
The document discusses the evolution of modern physics, beginning with classical physics in the 1890s and detailing key theories such as the kinetic theory of gases, conservation laws, and the atomic theory of matter. It highlights pivotal contributions from notable physicists including Galileo, Newton, and Maxwell, emphasizing the development of fundamental concepts in mechanics, electromagnetism, and thermodynamics. Ultimately, it outlines unresolved questions and the transition to modern physics, particularly the introduction of relativity and quantum mechanics in response to new discoveries and complexities.