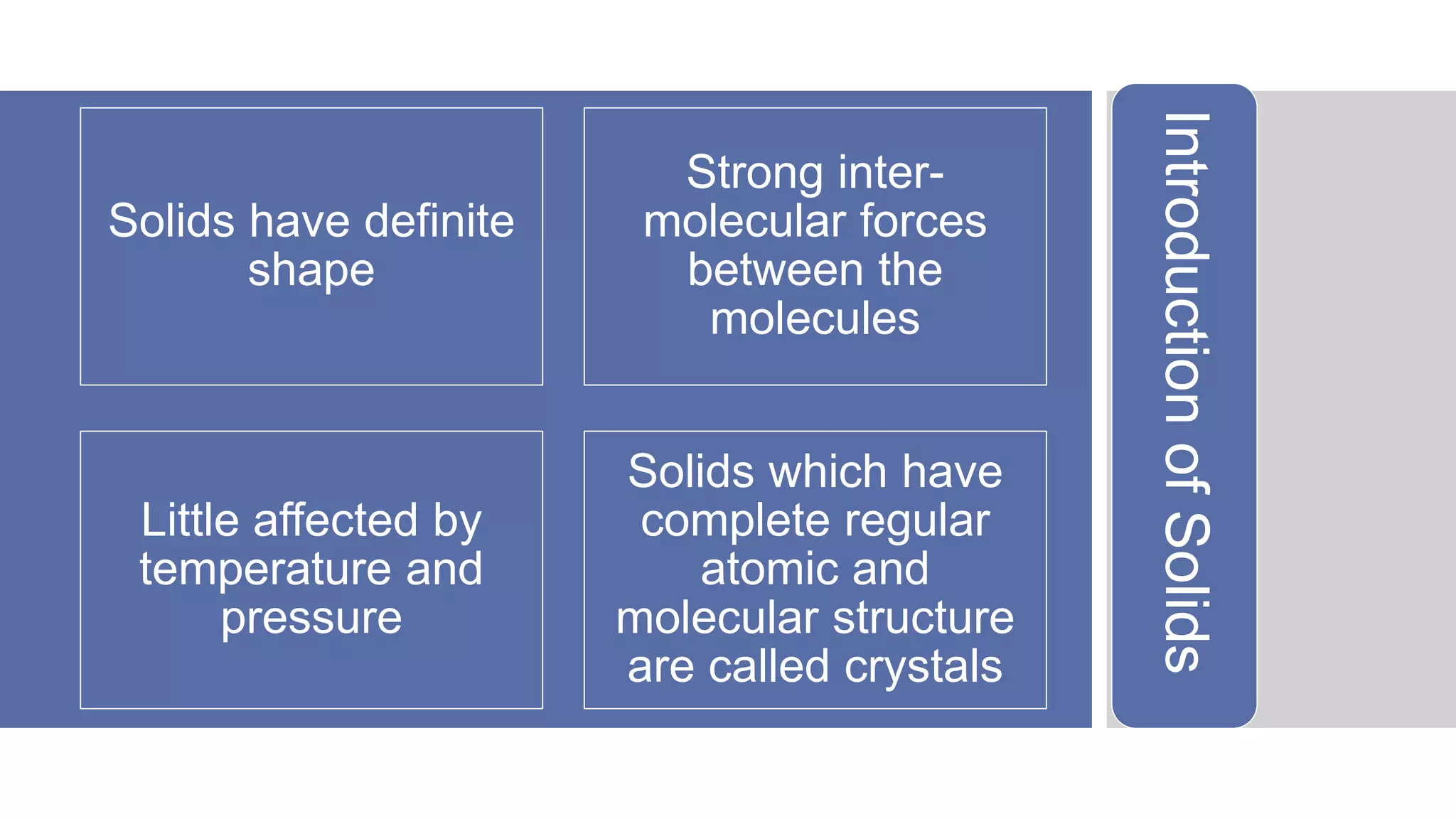
The document discusses various properties of crystalline solids including:
- Anisotropy refers to substances that have different properties in different directions due to different arrangements of crystals.
- Isomorphism describes different substances with the same crystal shape due to same atomic ratios.
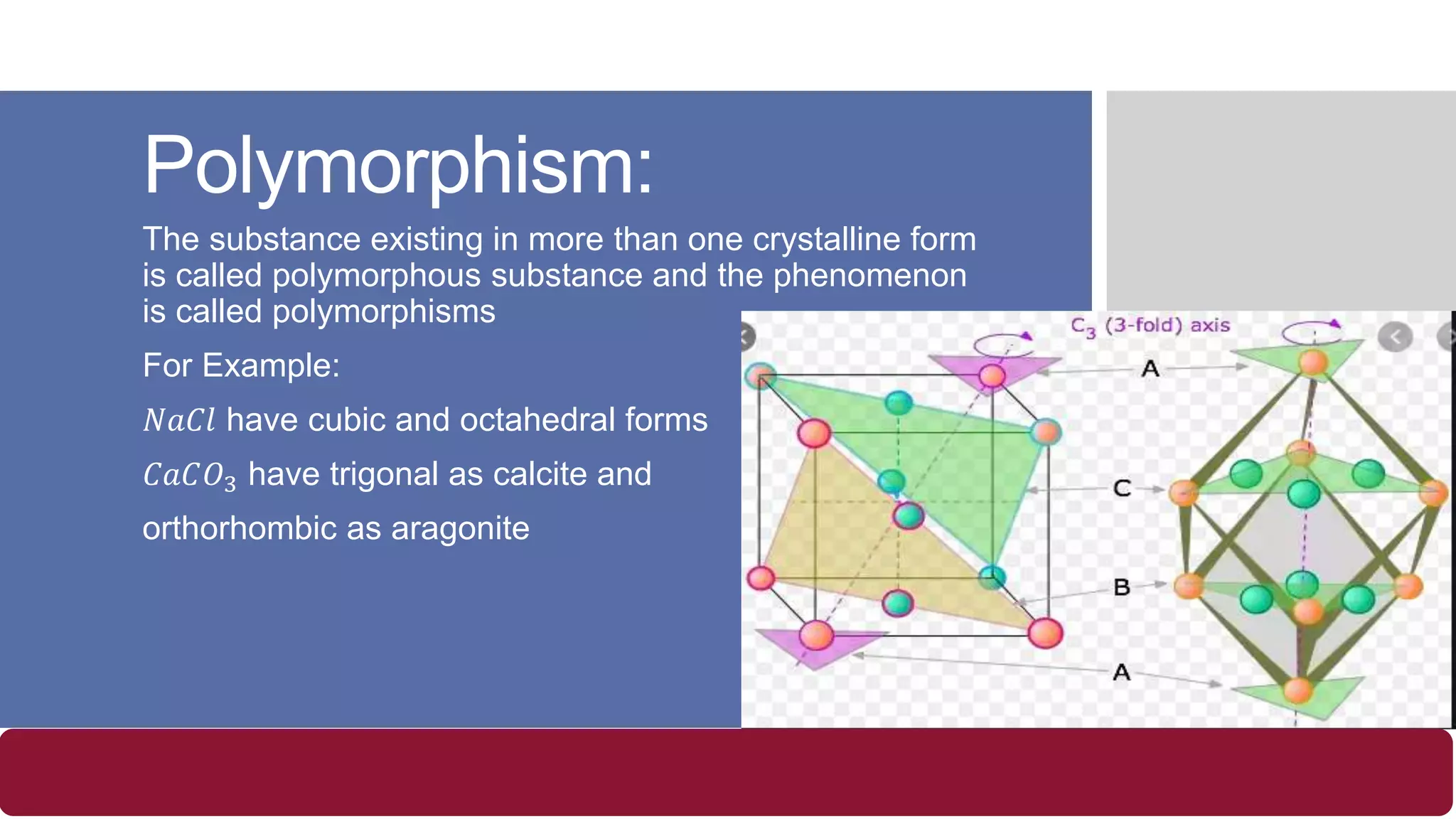
- Polymorphism is when a substance exists in more than one crystalline form.
- Allotropy is when an element exists in different crystalline forms called allotropes.
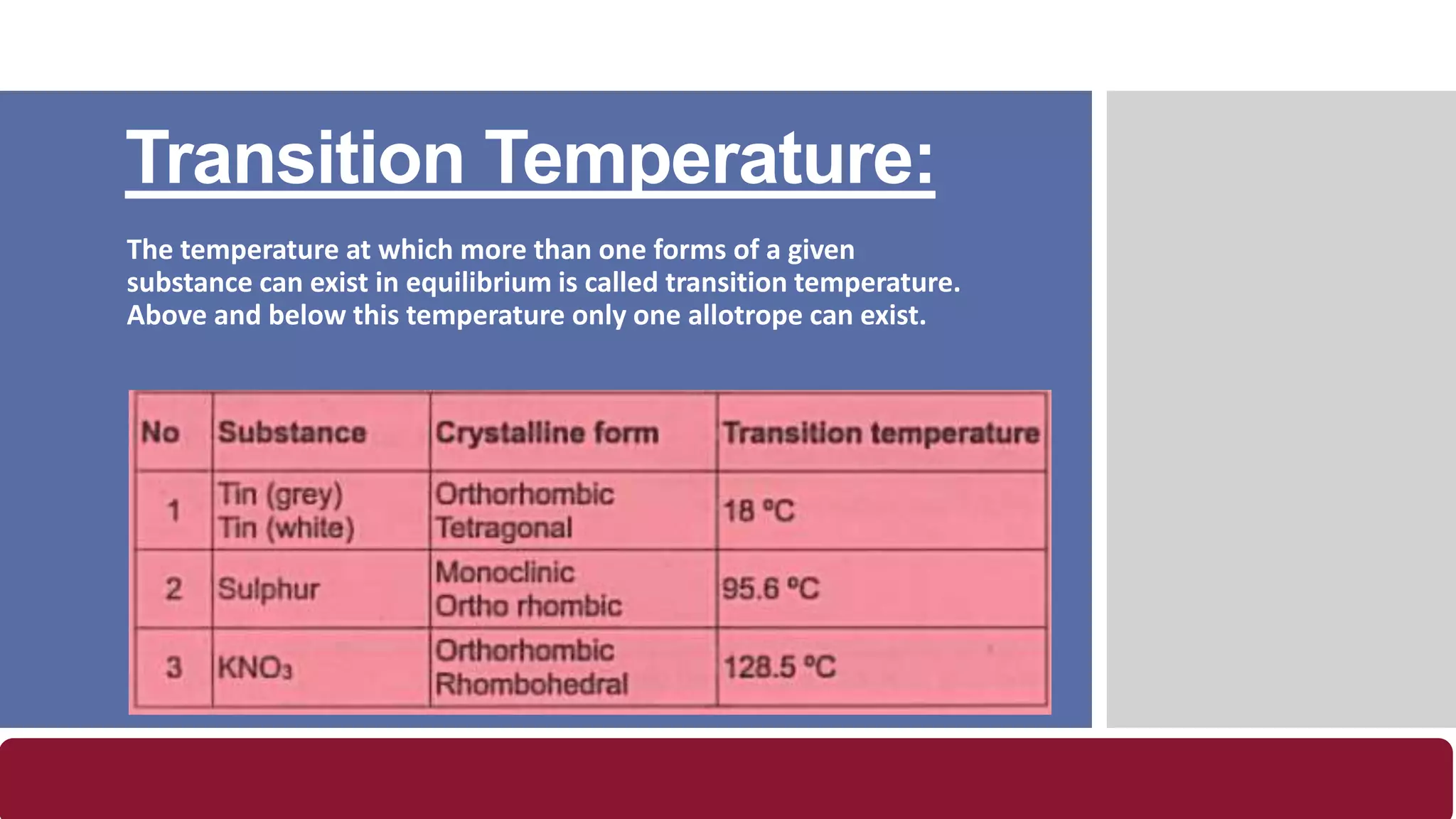
- Transition temperature is when more than one form of a substance can exist in equilibrium.
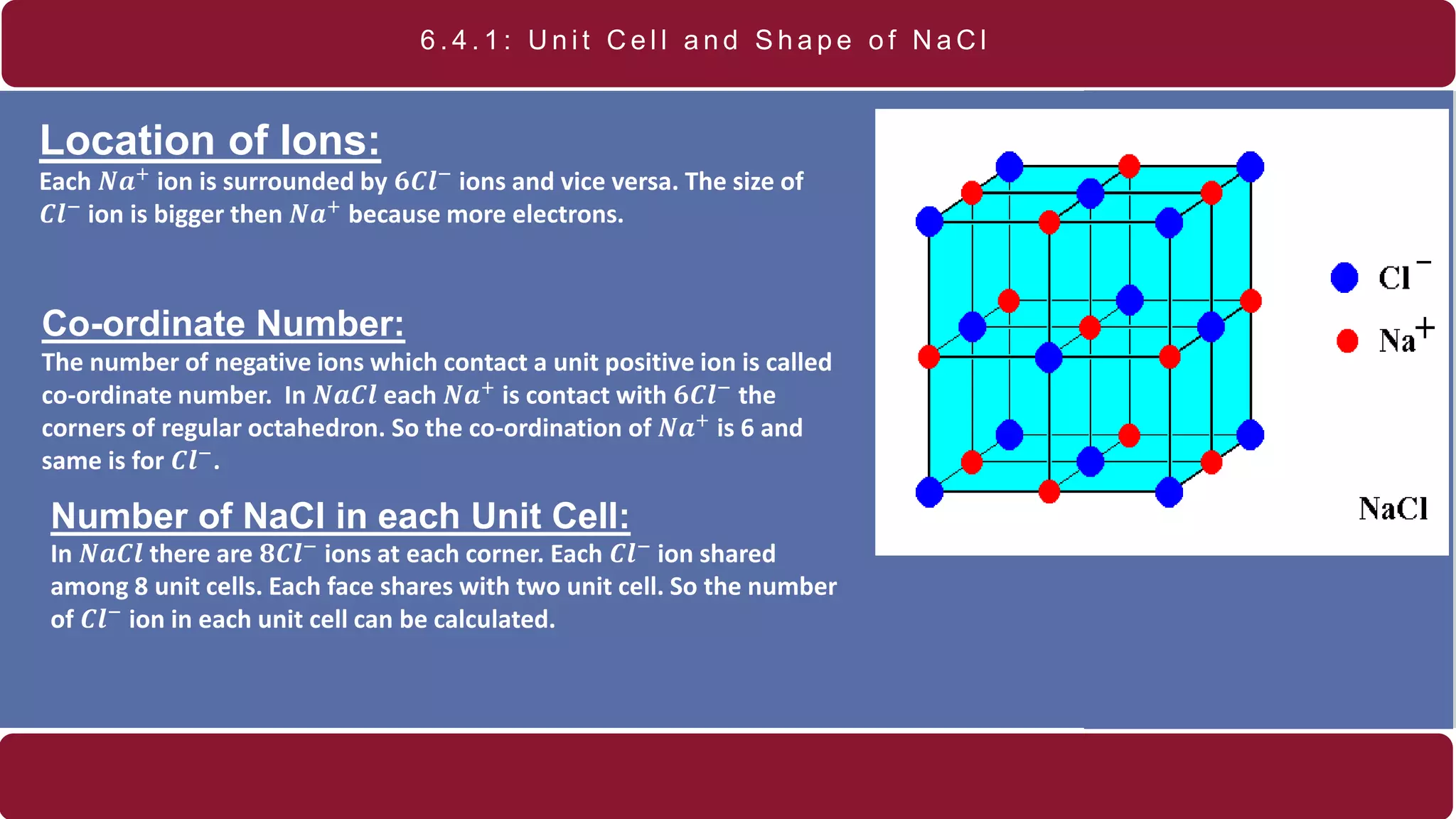
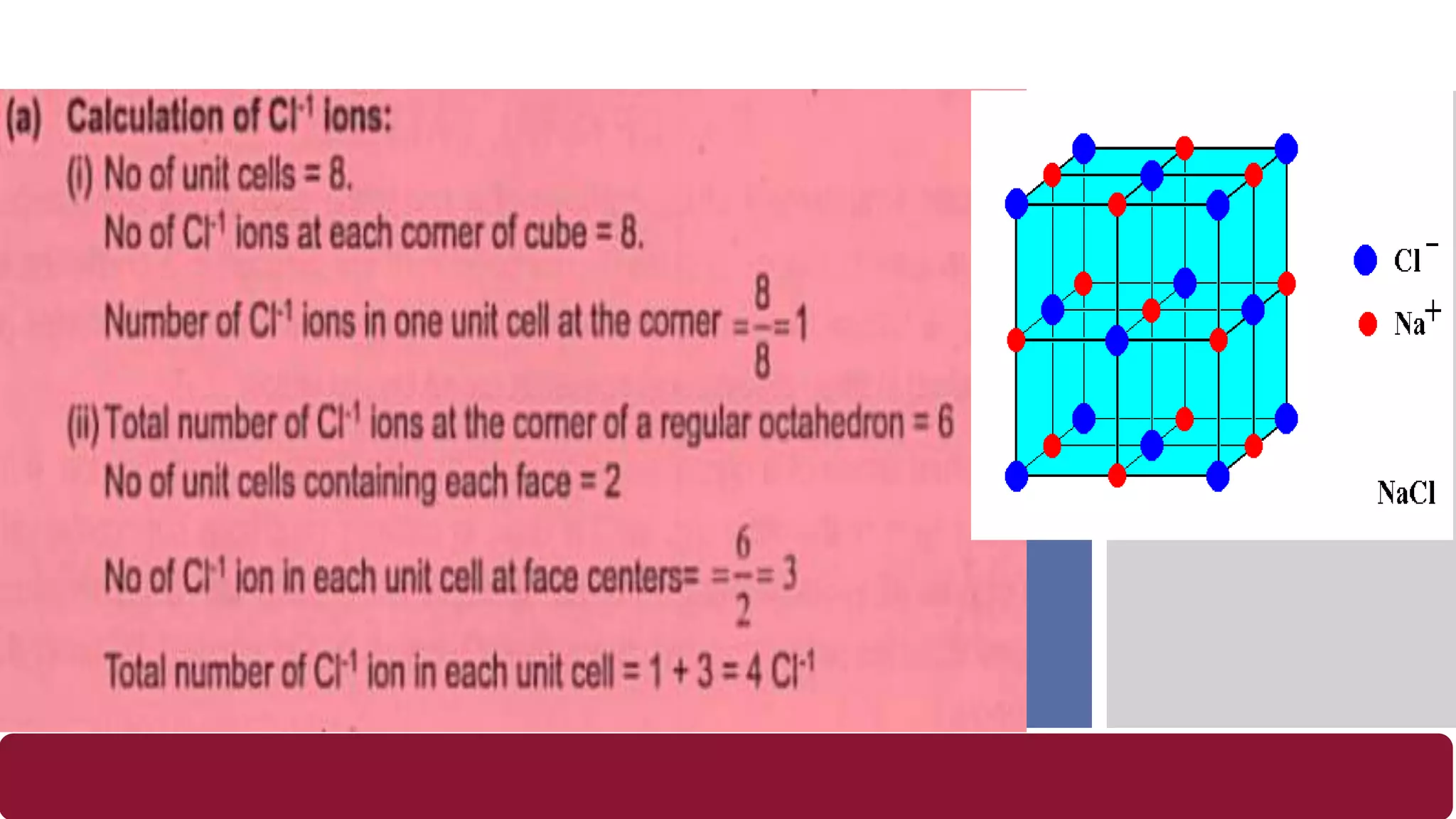
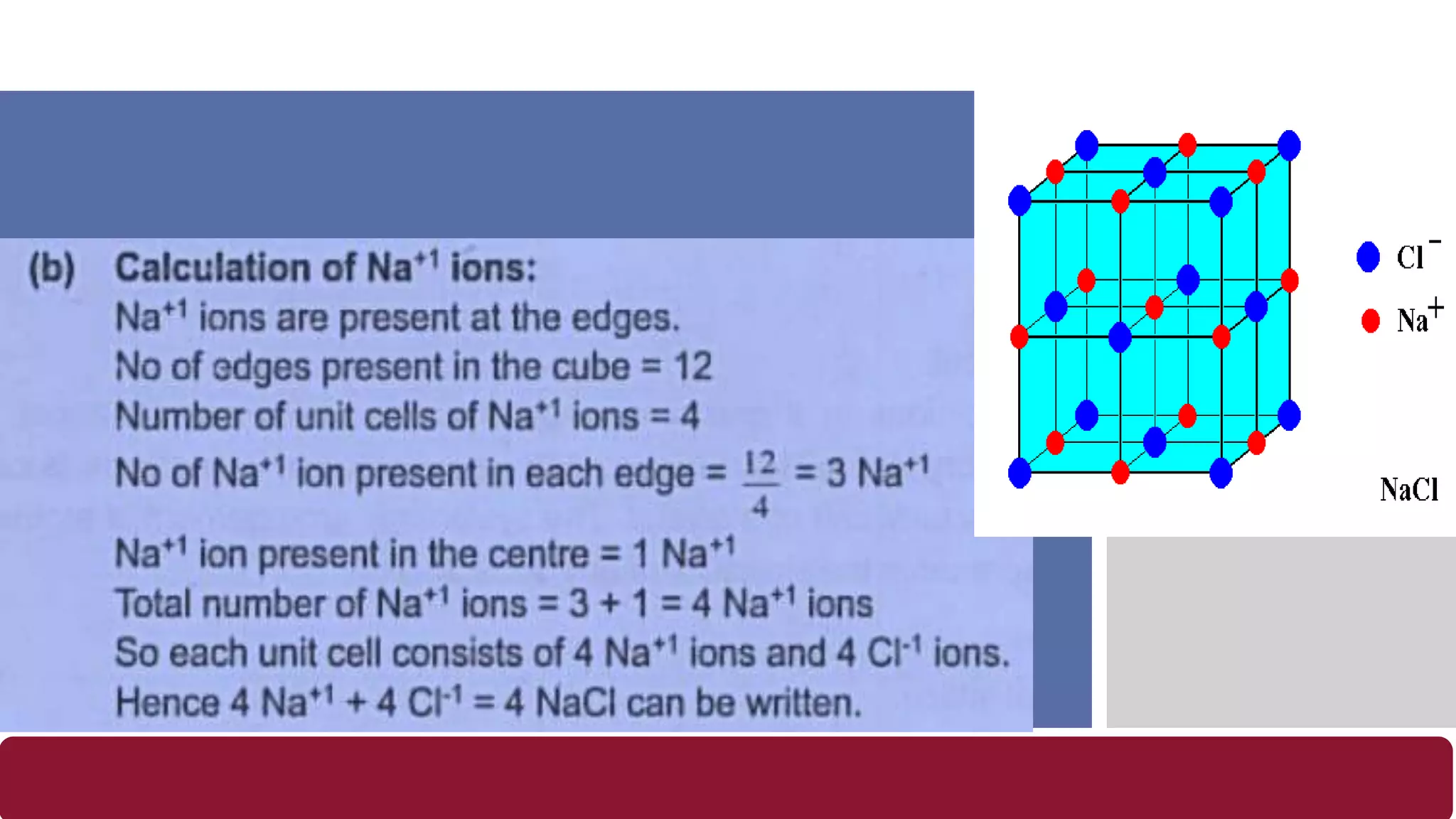
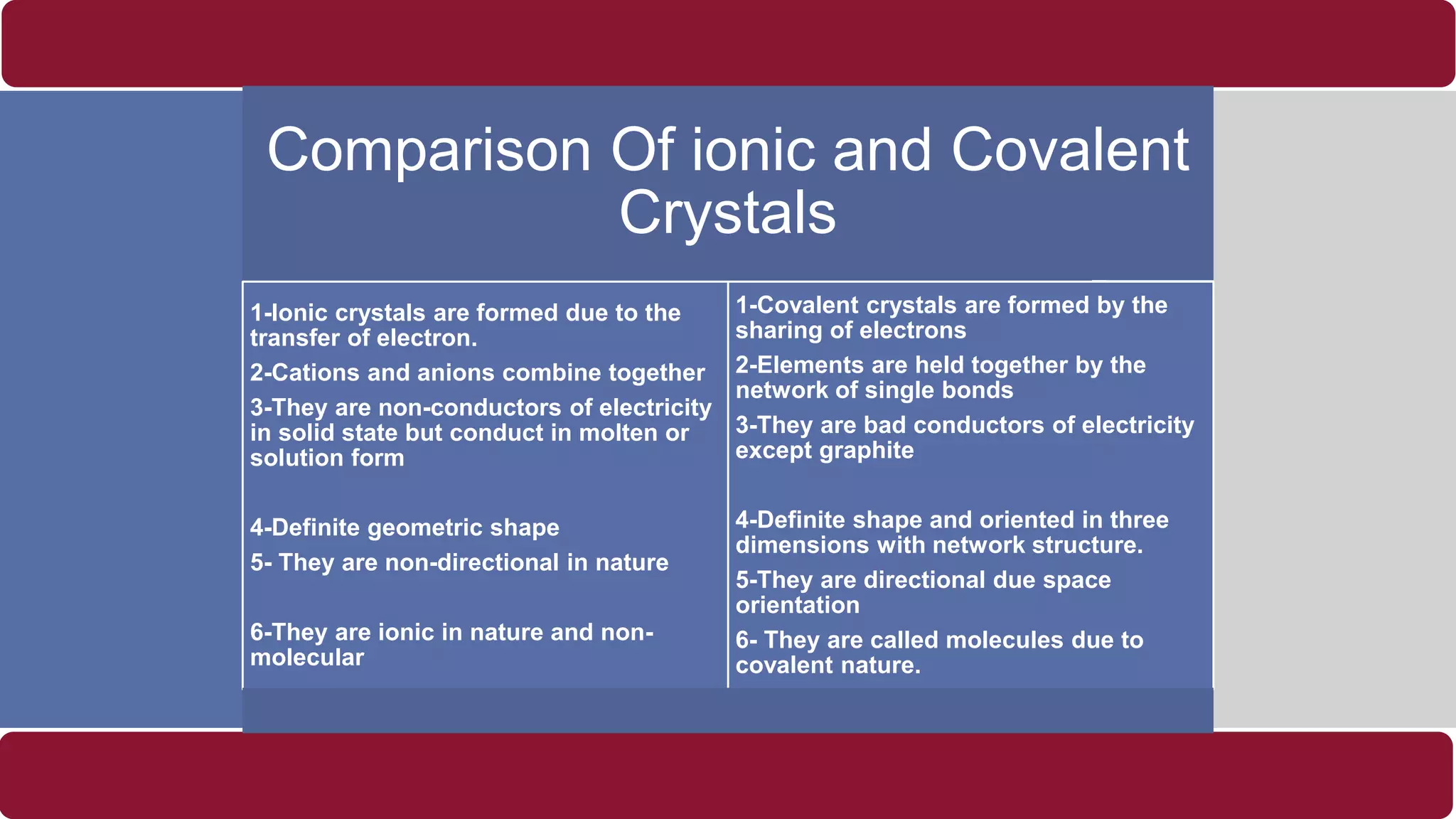
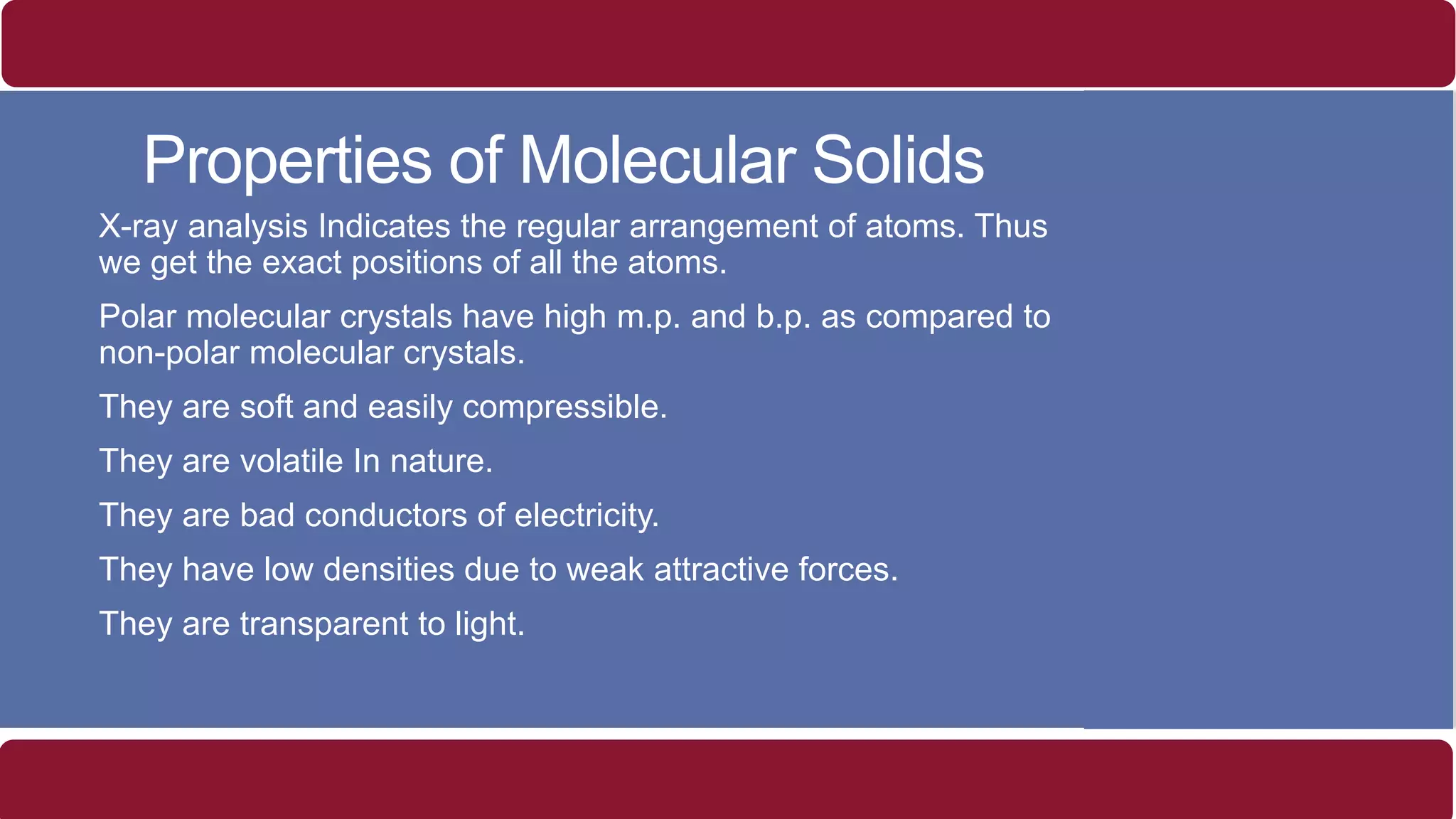
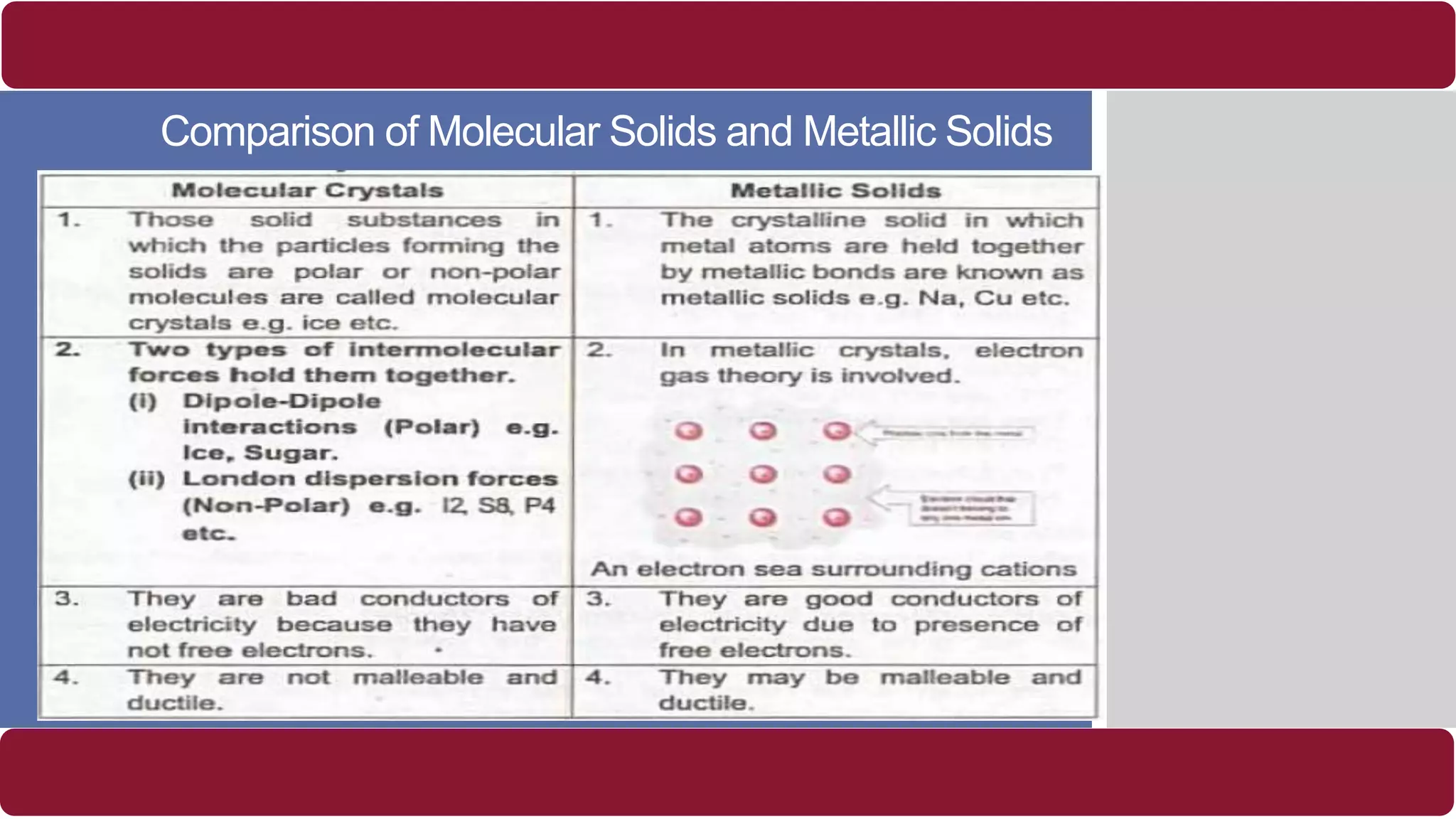
It also discusses crystal lattices, unit cells, ionic bonding in NaCl, lattice energy, properties of ionic and covalent crystals, molecular solids, and