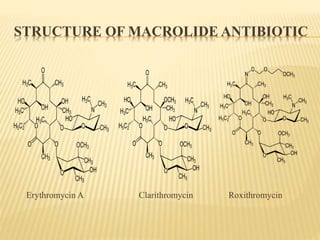
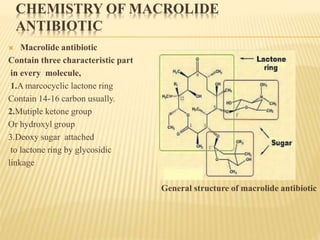
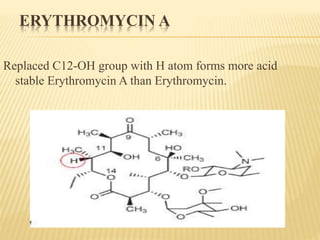
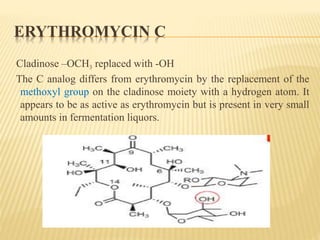
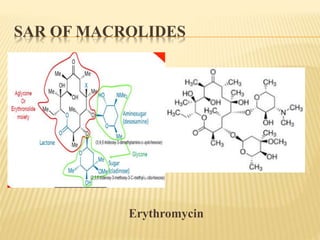
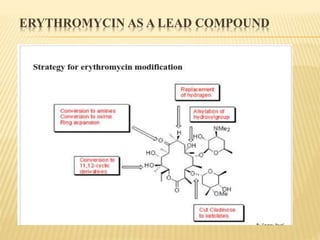
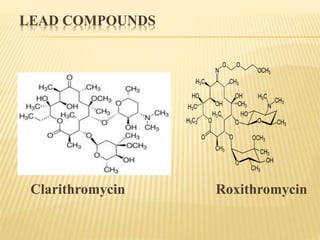
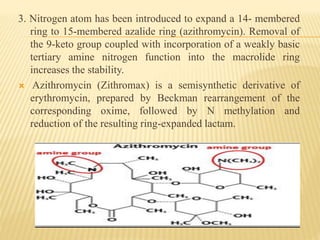
This document discusses macrolide antibiotics. It begins by introducing macrolides as a class of antibiotics characterized by their large lactone ring structures. It then classifies macrolides based on their ring size and discusses key macrolides like erythromycin. The document outlines the chemistry of macrolides, including their characteristic lactone ring, ketone/hydroxyl groups, and deoxy sugar components. It also discusses the structural activity relationship of macrolides using erythromycin as a lead compound and how derivatives like clarithromycin, roxithromycin and azithromycin were developed for improved properties.