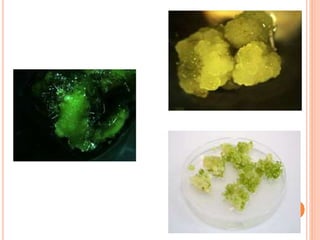
Secondary metabolites are organic compounds produced by plant metabolism that are not essential for growth or reproduction but provide other benefits. They often function in plant defense against herbivores and pathogens. There are several types of plant tissue cultures used to study secondary metabolism, including organized cultures of tissues, disorganized callus cultures, hairy root cultures, and immobilized cell cultures where cells are confined within a matrix.