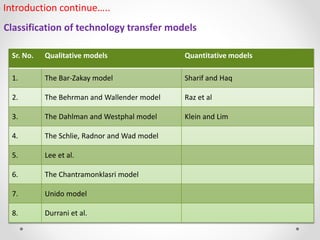
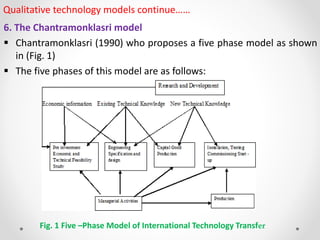
1. The document discusses qualitative and quantitative models of technology transfer. Qualitative models delineate activities and factors that influence success, while quantitative models aim to quantify parameters and minimize incompatibility between transferors and transferees.
2. Several qualitative models are described, including the Bar-Zakay, Behrman and Wallender, Dahlman and Westphal, and Schlie, Radnor and Wad models. These models outline stages of the technology transfer process and factors that influence effectiveness.
3. Quantitative models discussed aim to measure parameters like potential technological distance between transferors and transferees, and examine the technological "catch-up" process when a leader assists a follower.