This document discusses documentation, distribution, and electronic data handling procedures for pharmaceutical companies. It contains the following key points:
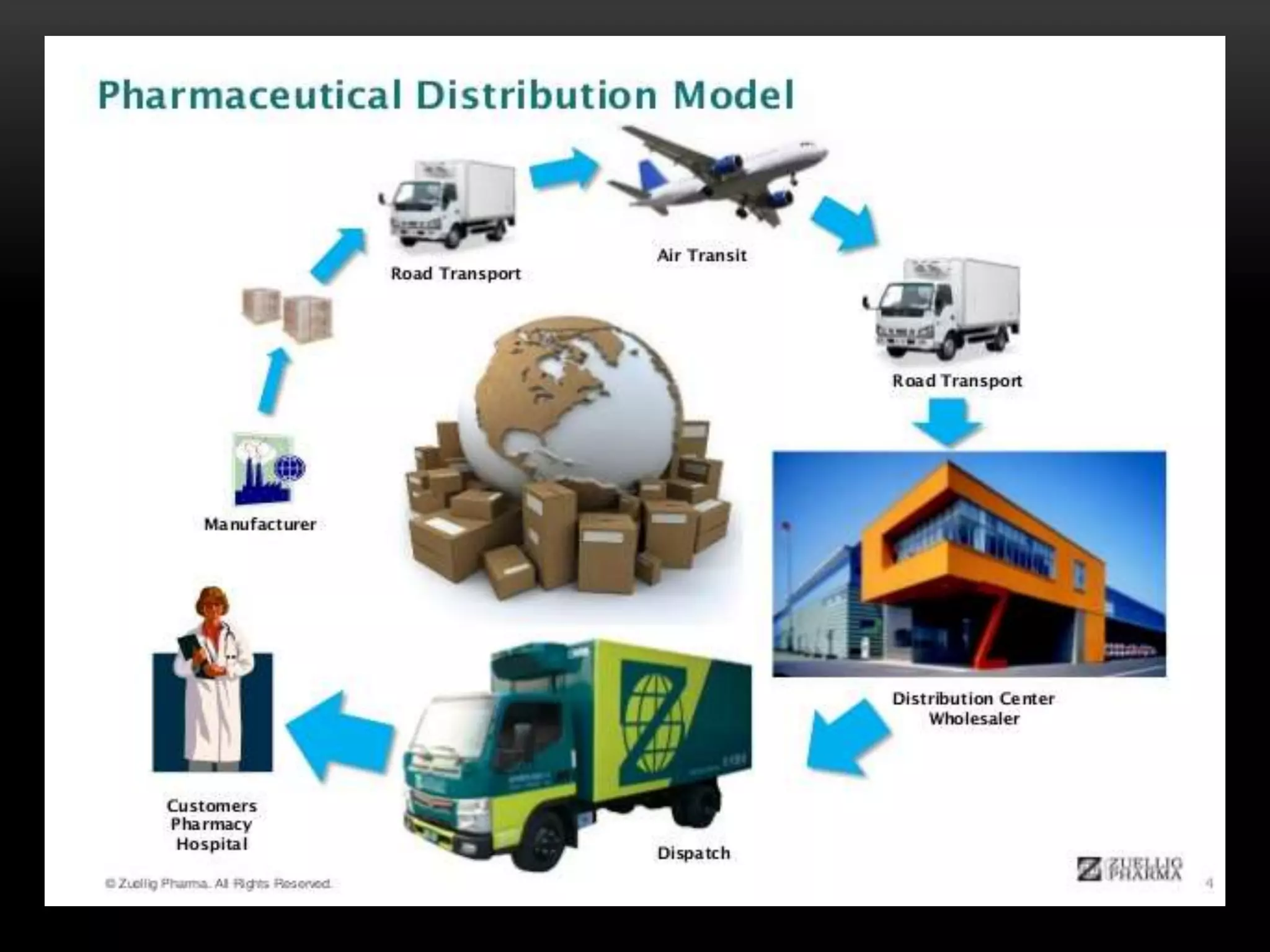
1. Documentation procedures ensure standardized and unambiguous procedures are followed to minimize errors. Distribution records must allow for efficient product recall if needed.
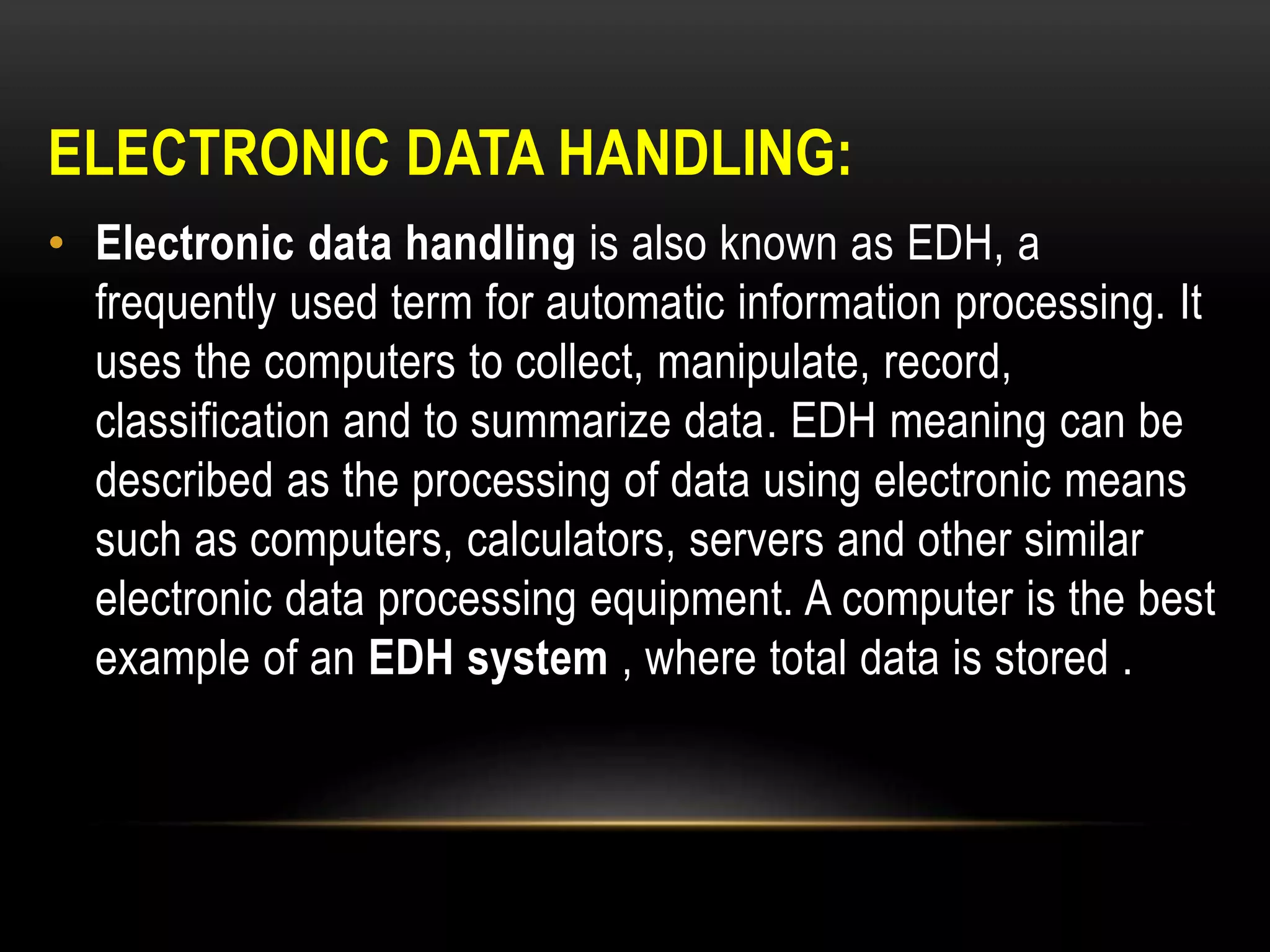
2. Electronic data handling uses computers to collect, store, and analyze data. It generates audit trails and records for activities like batch production. The ALCOA principles provide guidelines for ensuring electronic data is attributable, legible, contemporaneous, original, and accurate.
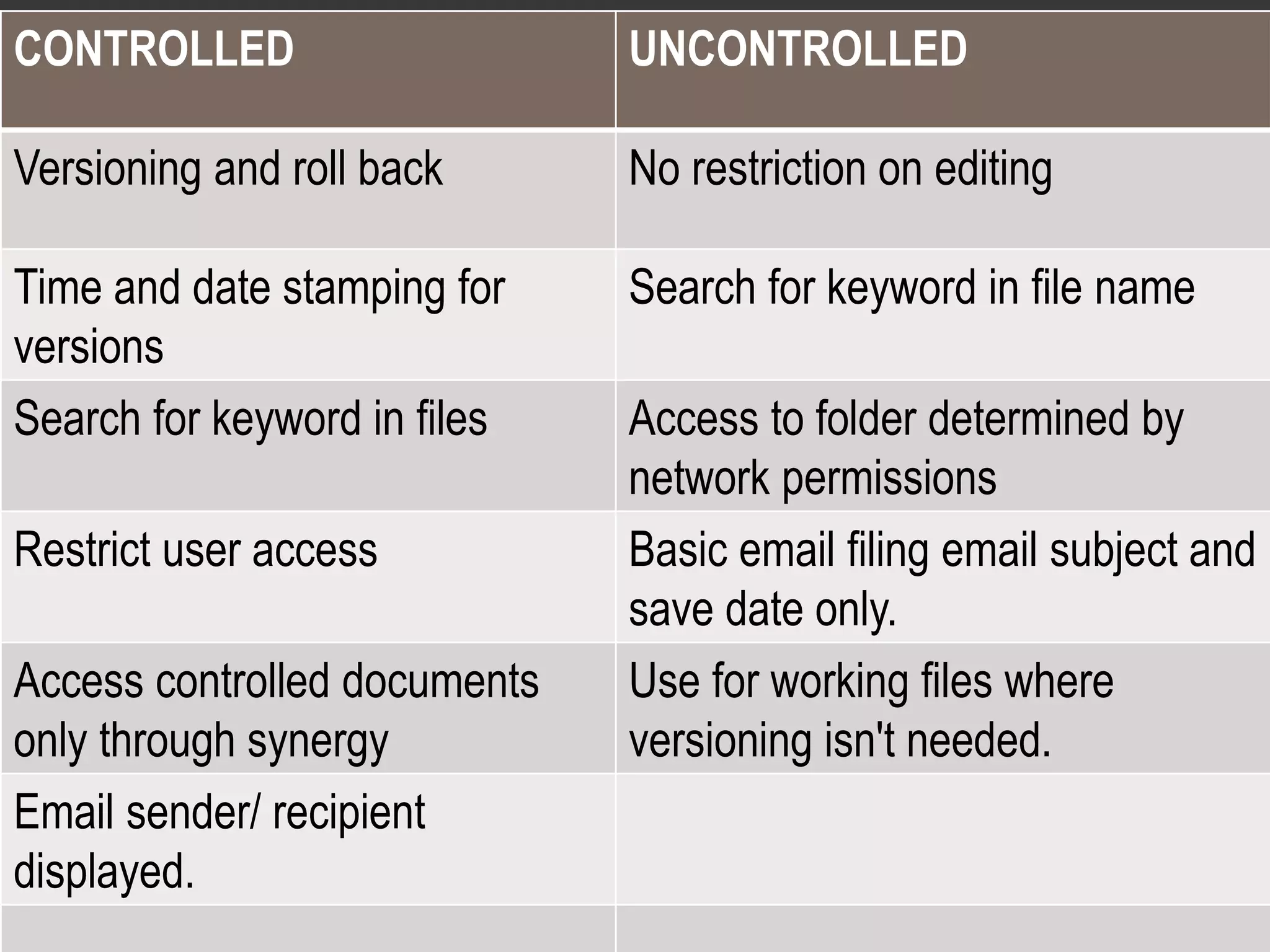
3. Controlled documents are subject to versioning and access restrictions to ensure only the correct version is used. Uncontrolled documents are not versioned and can be freely edited for reference purposes.