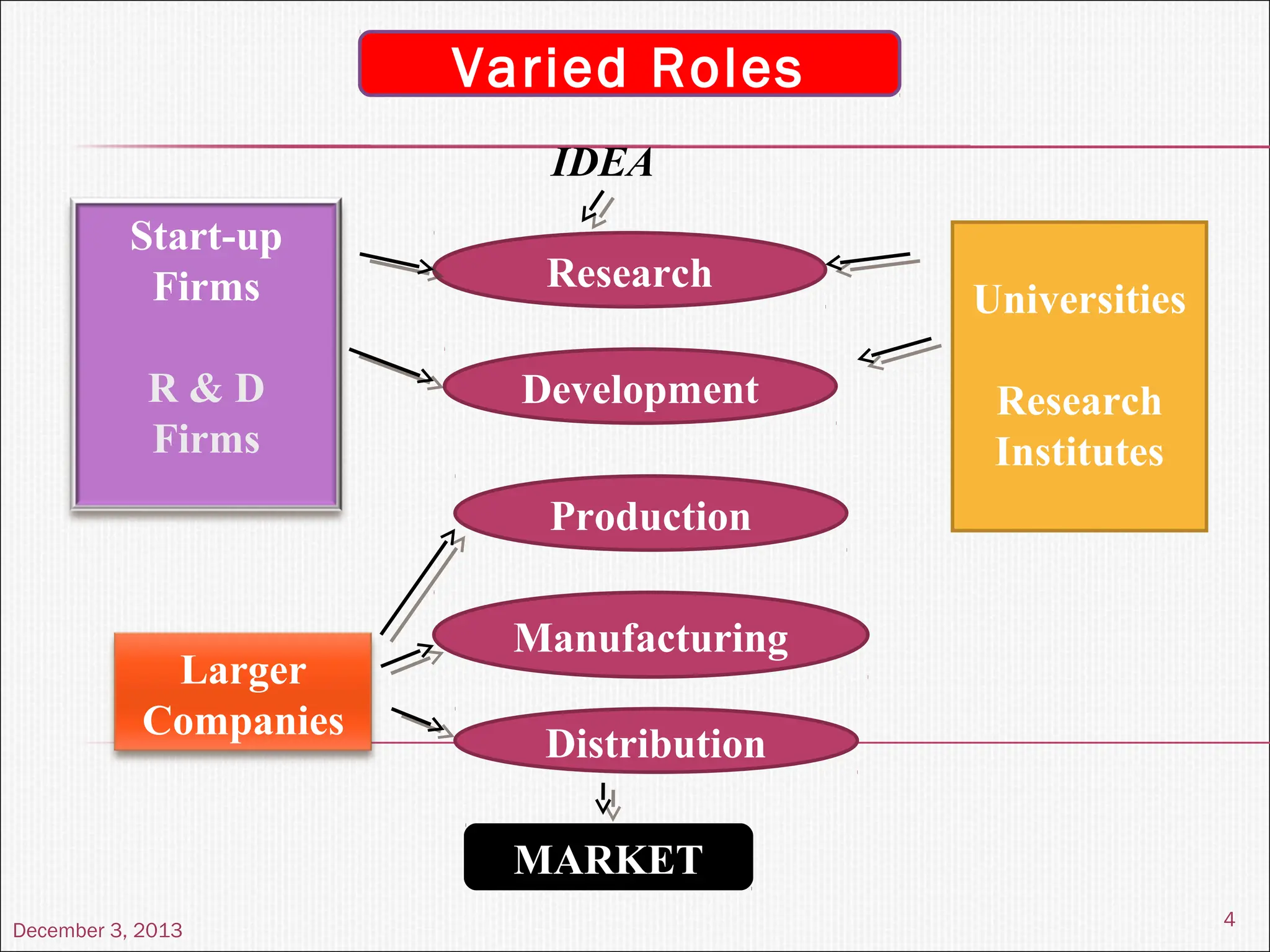
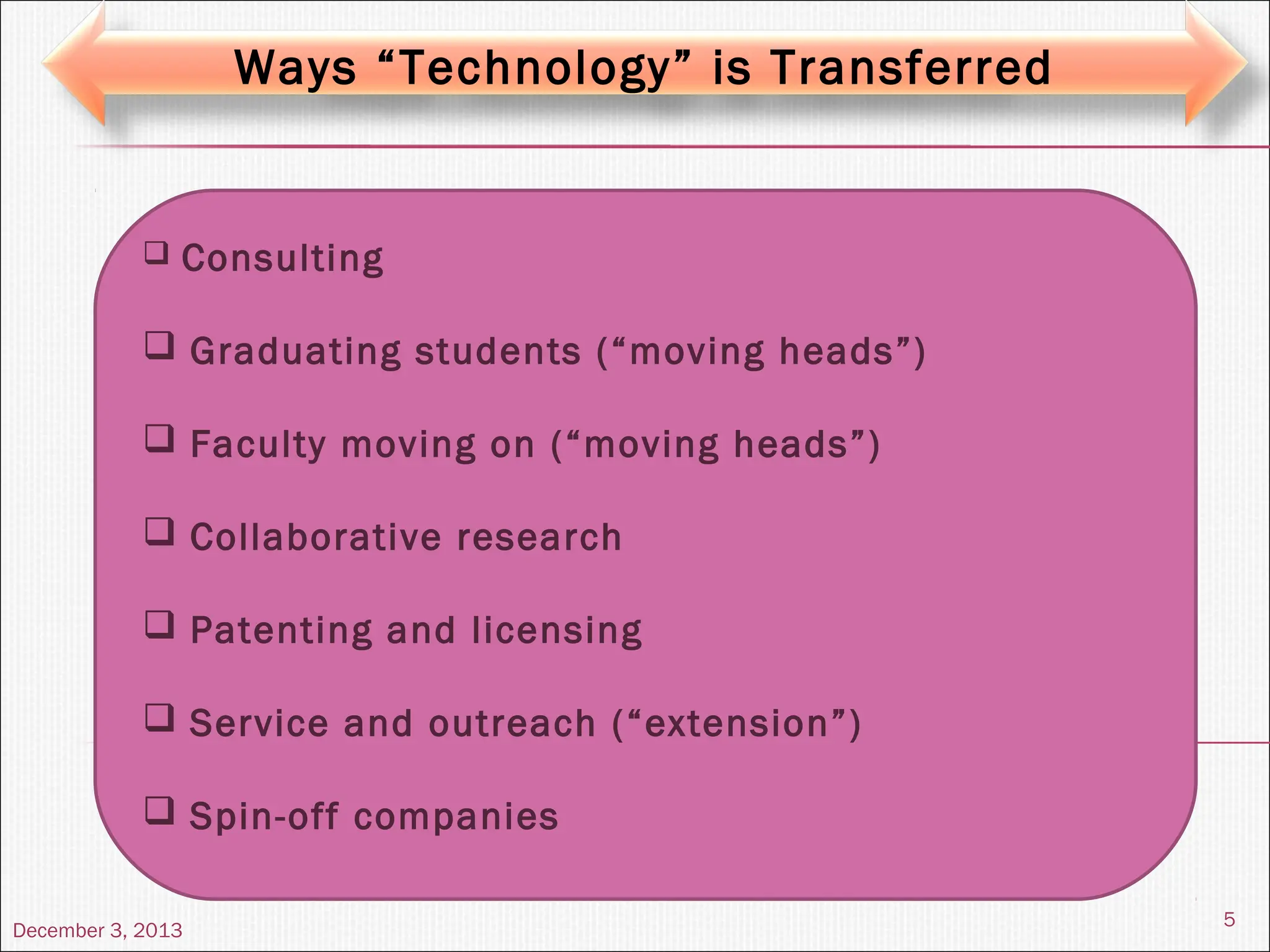
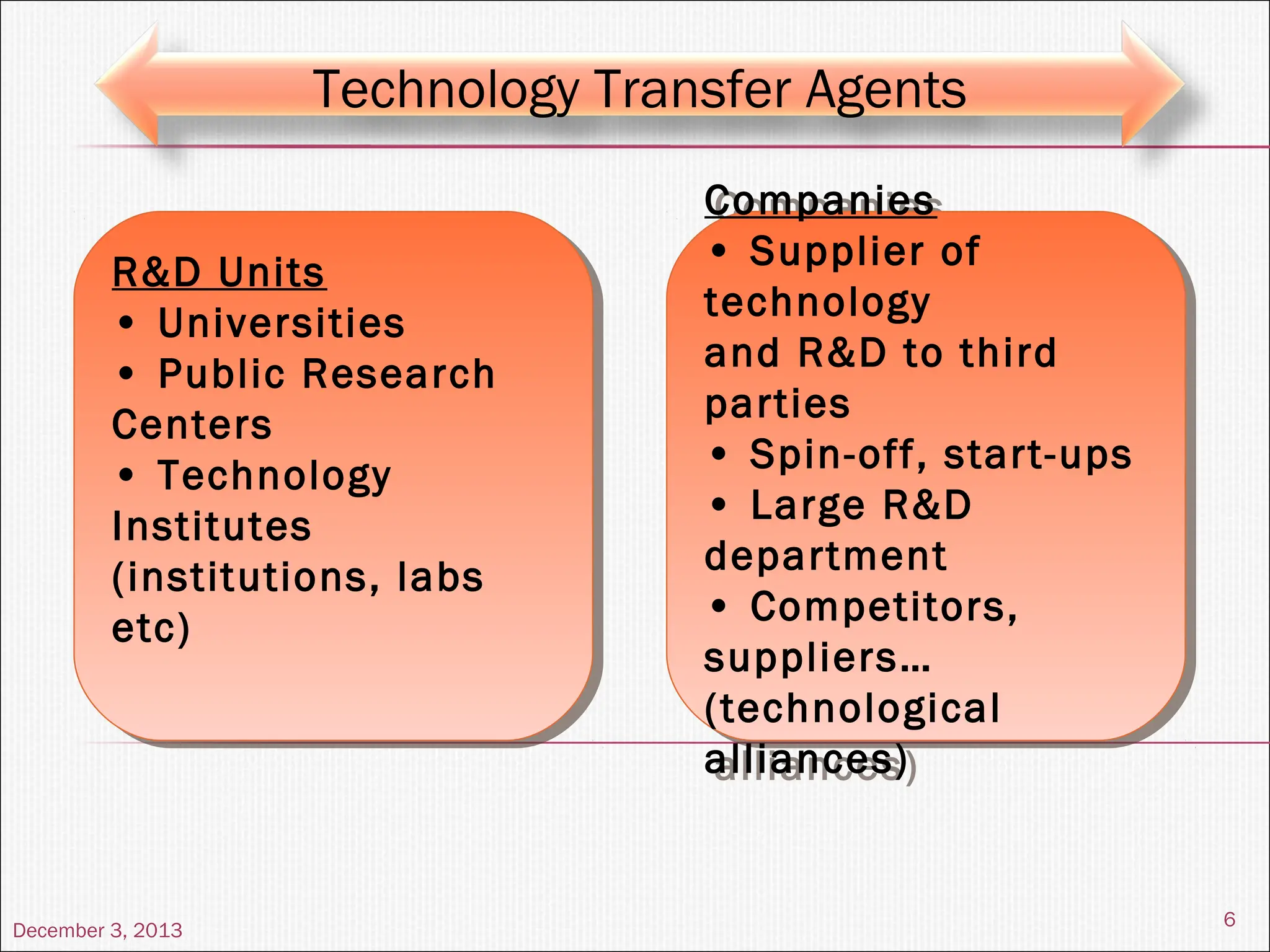
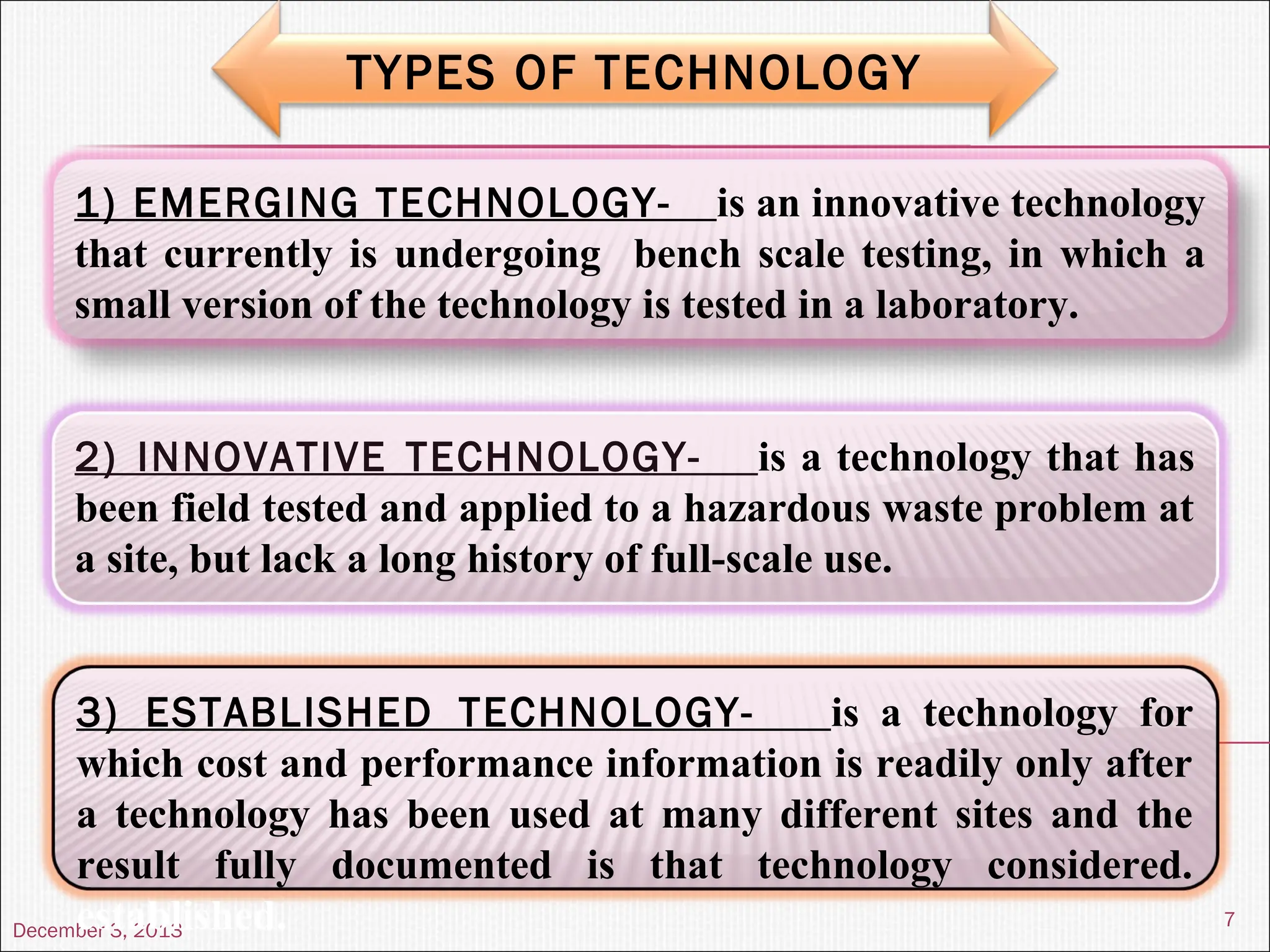
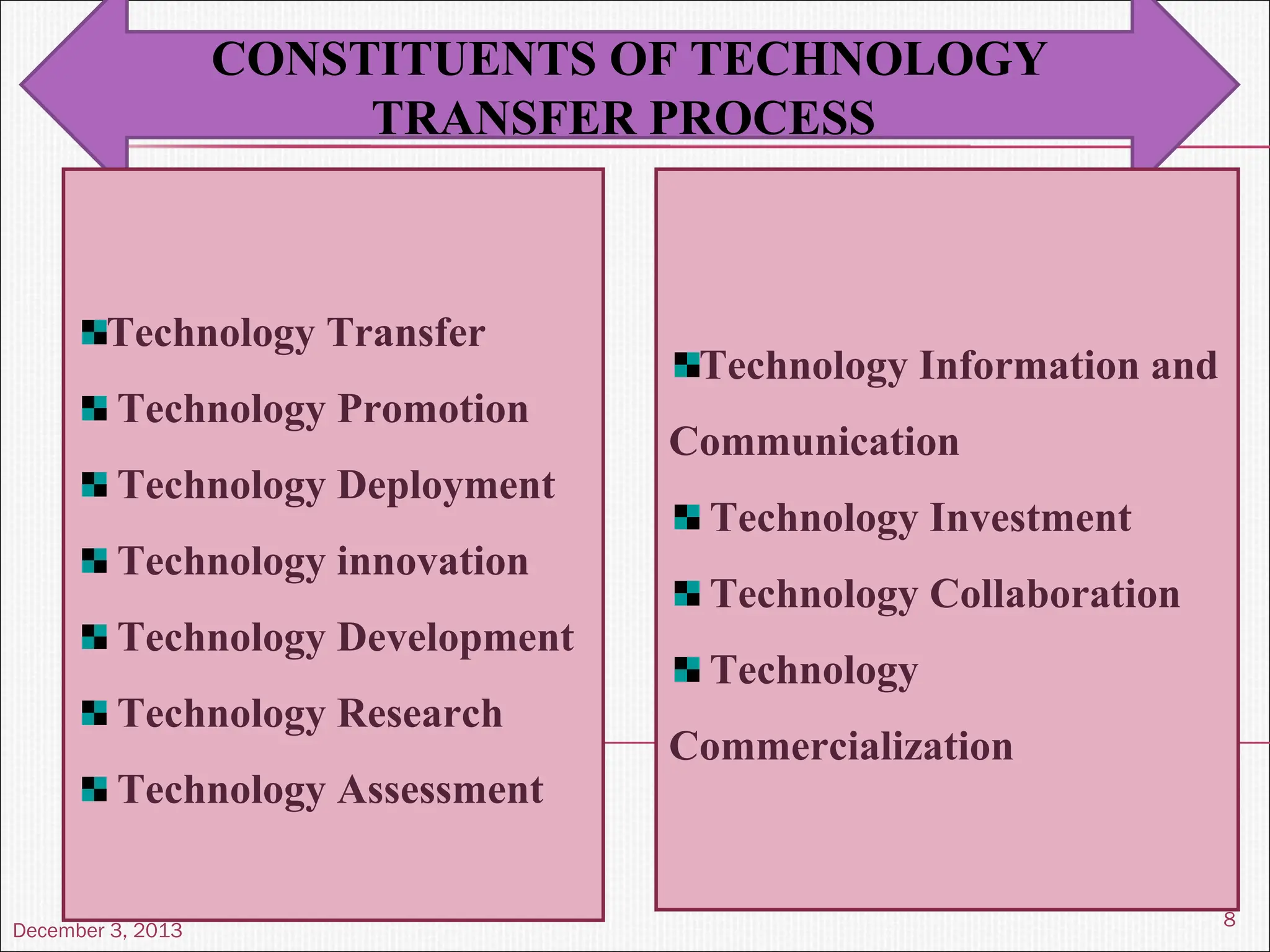
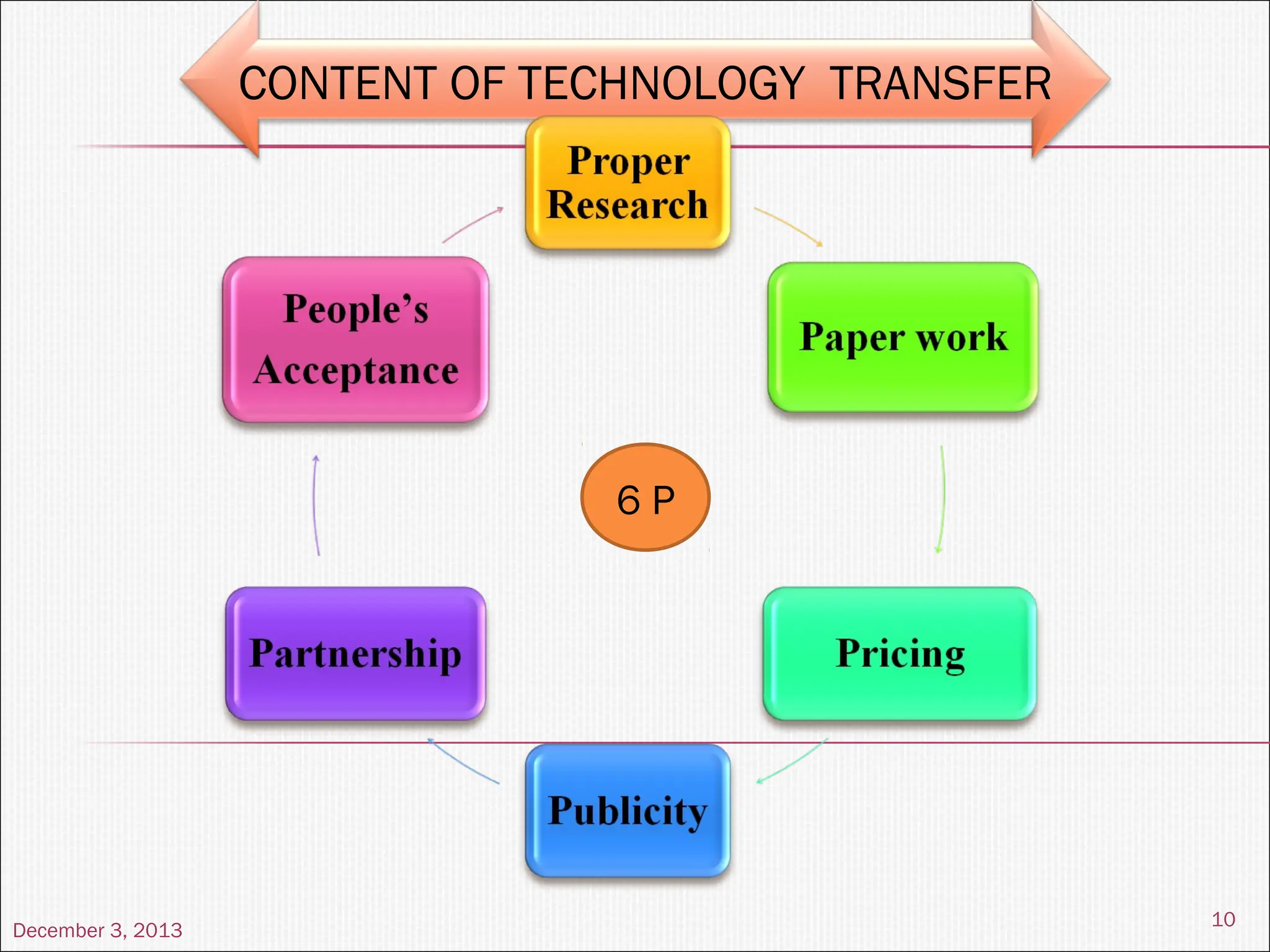
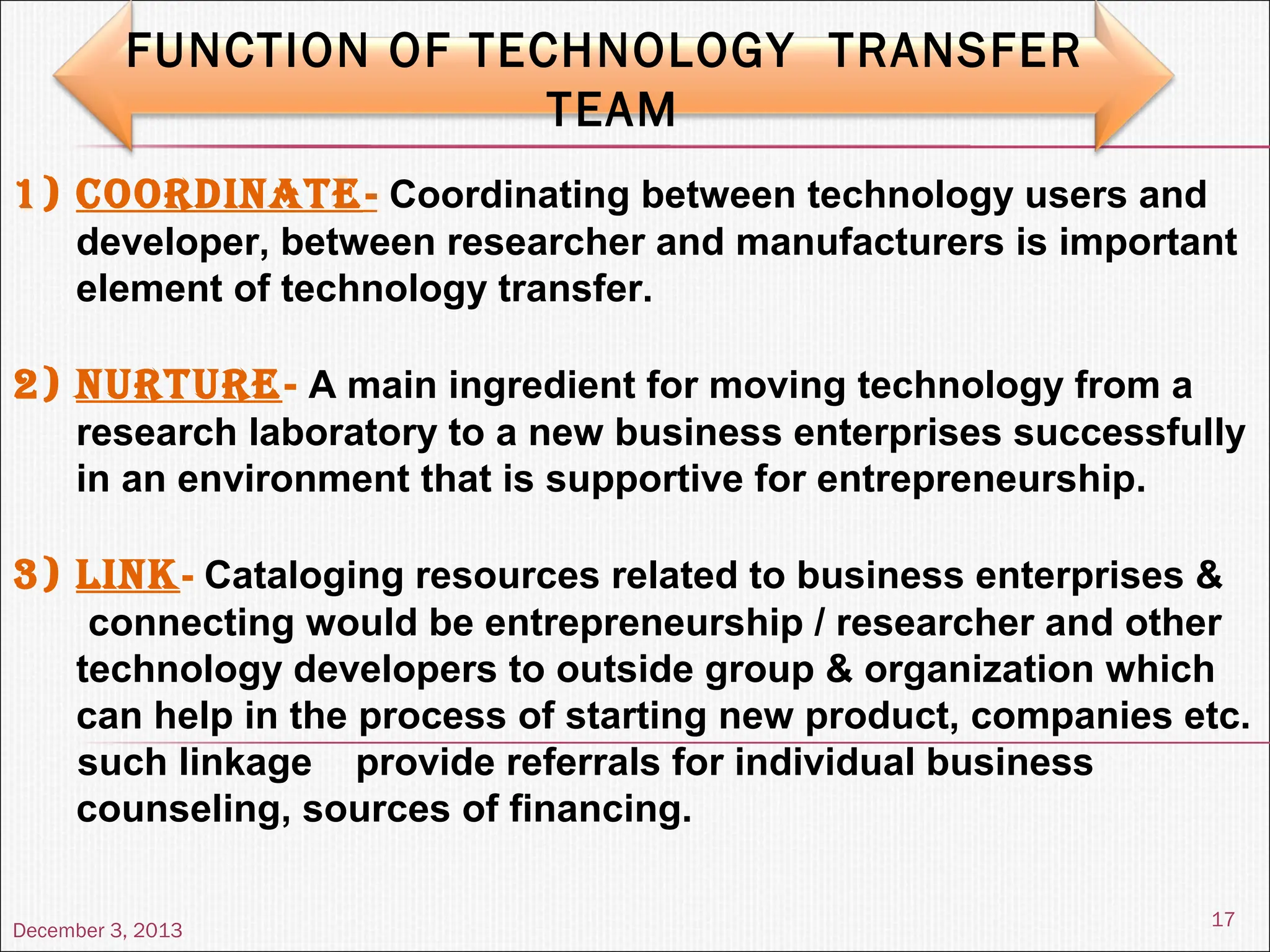
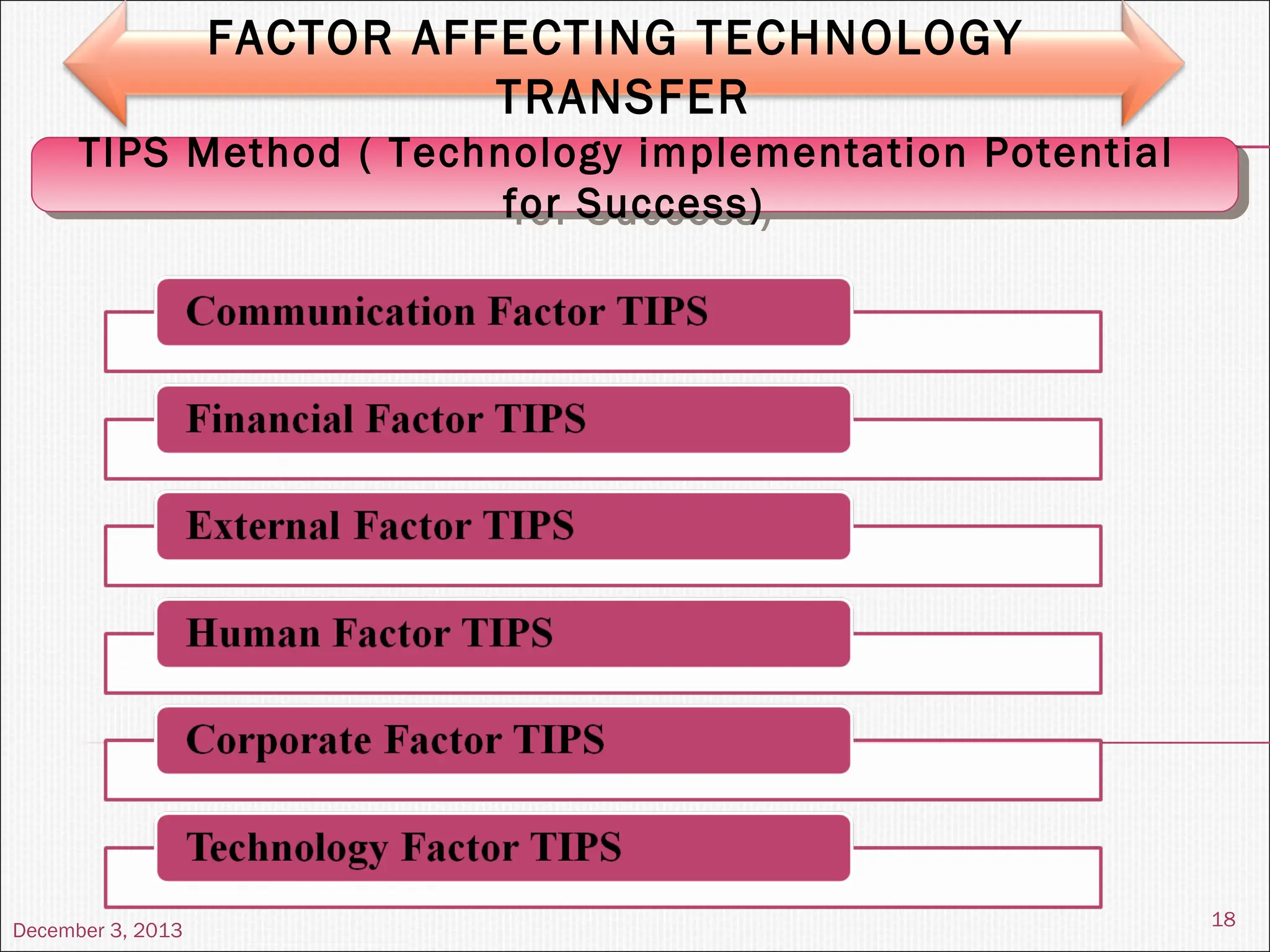
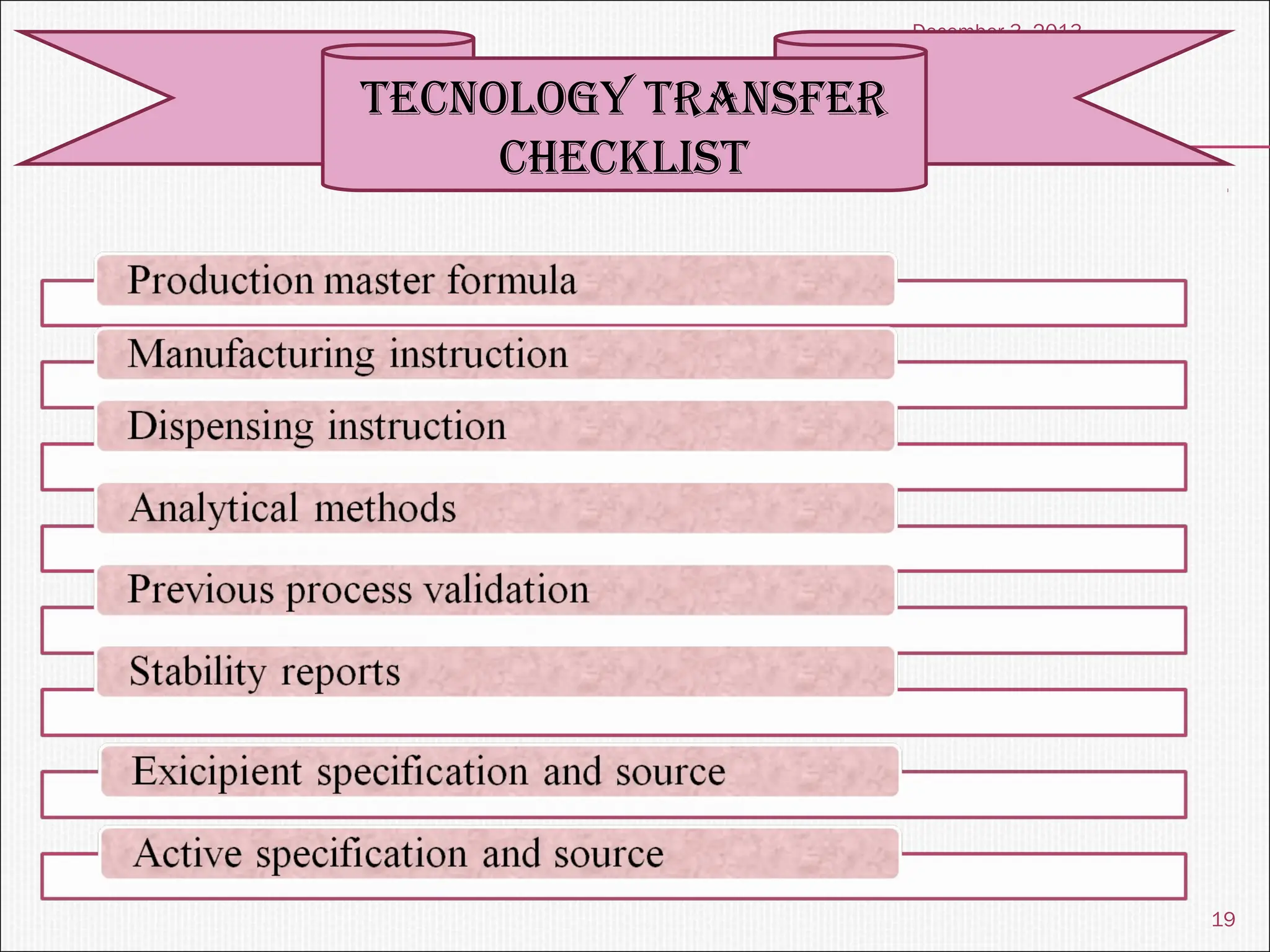
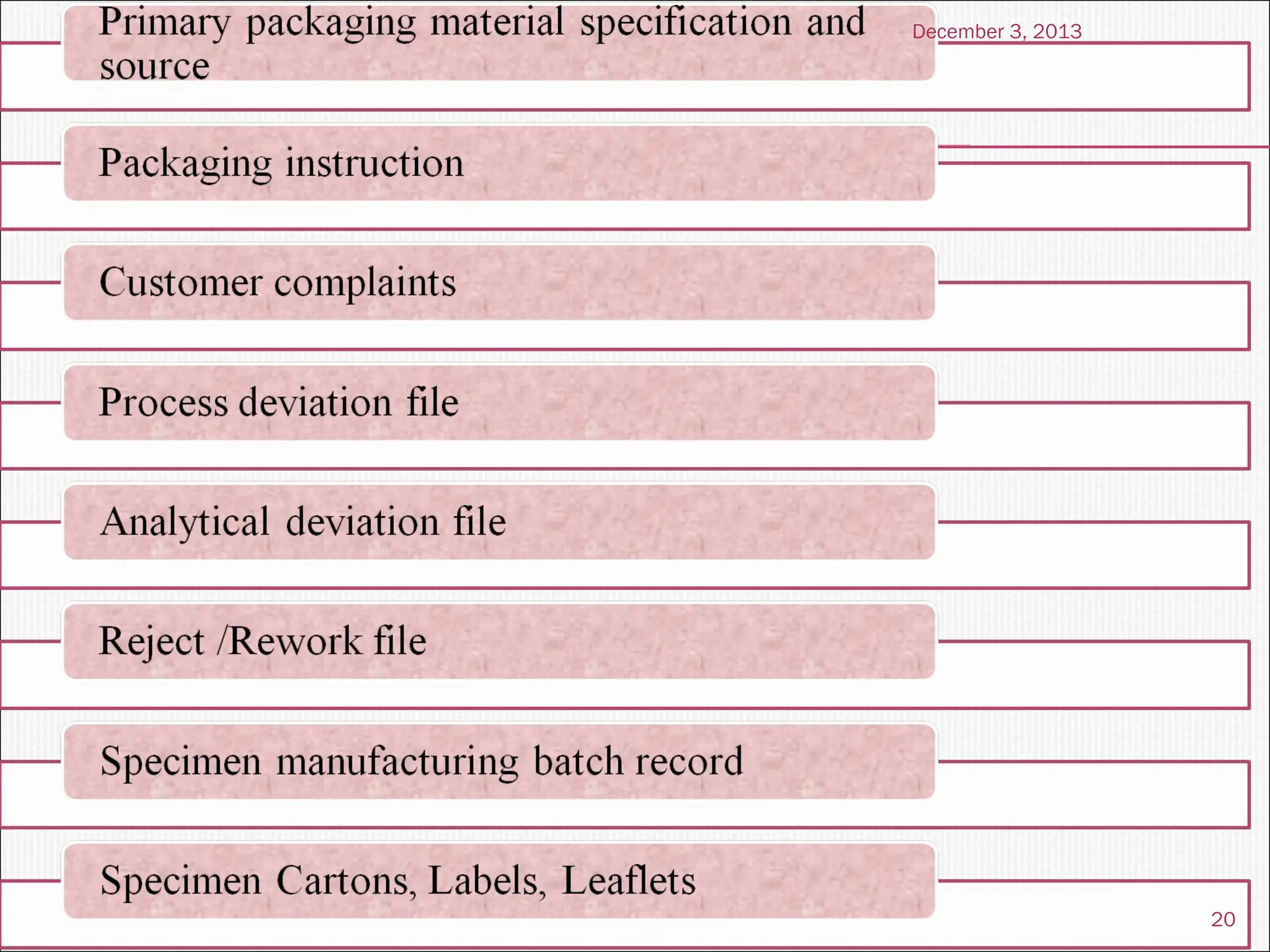
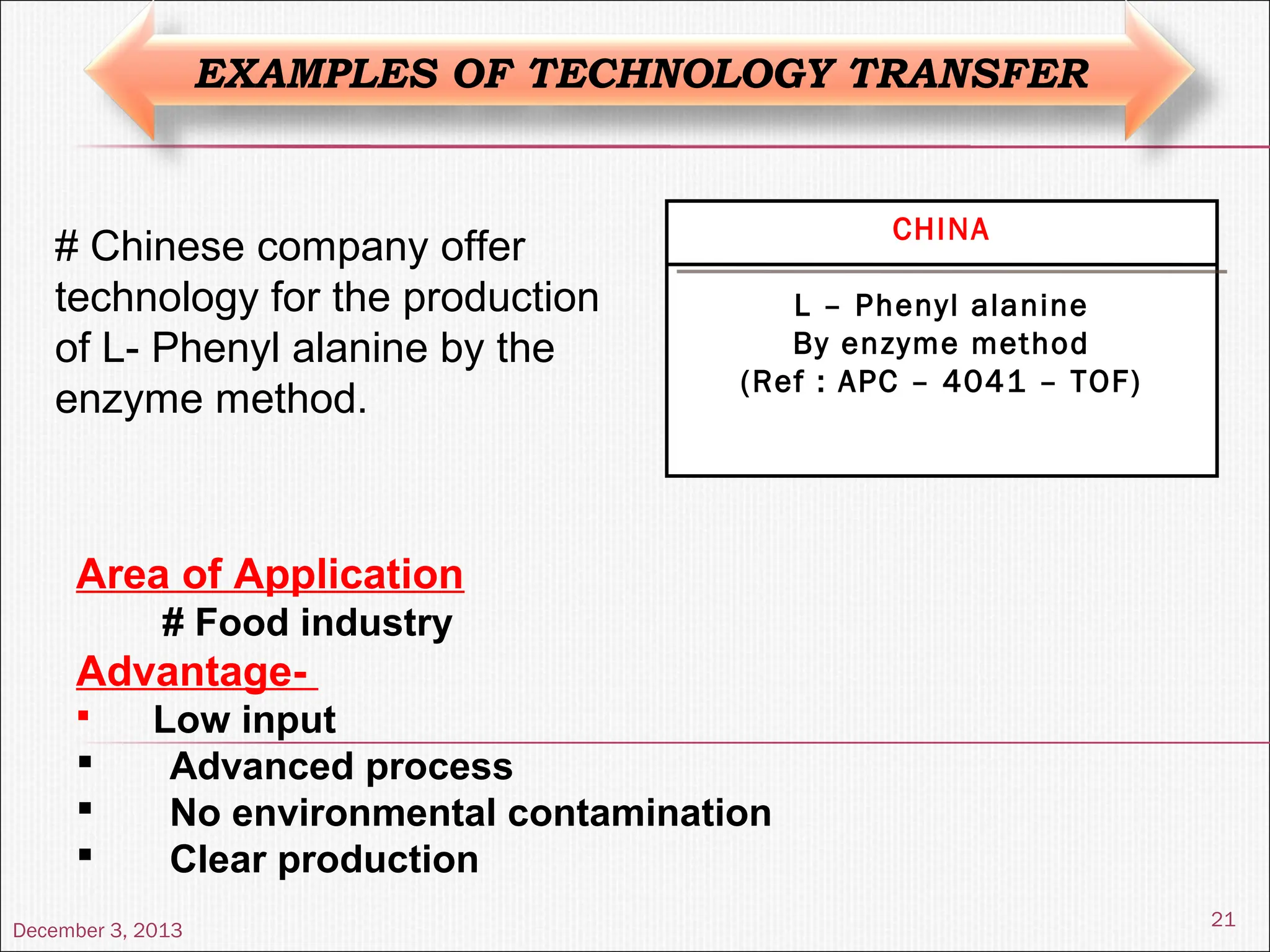
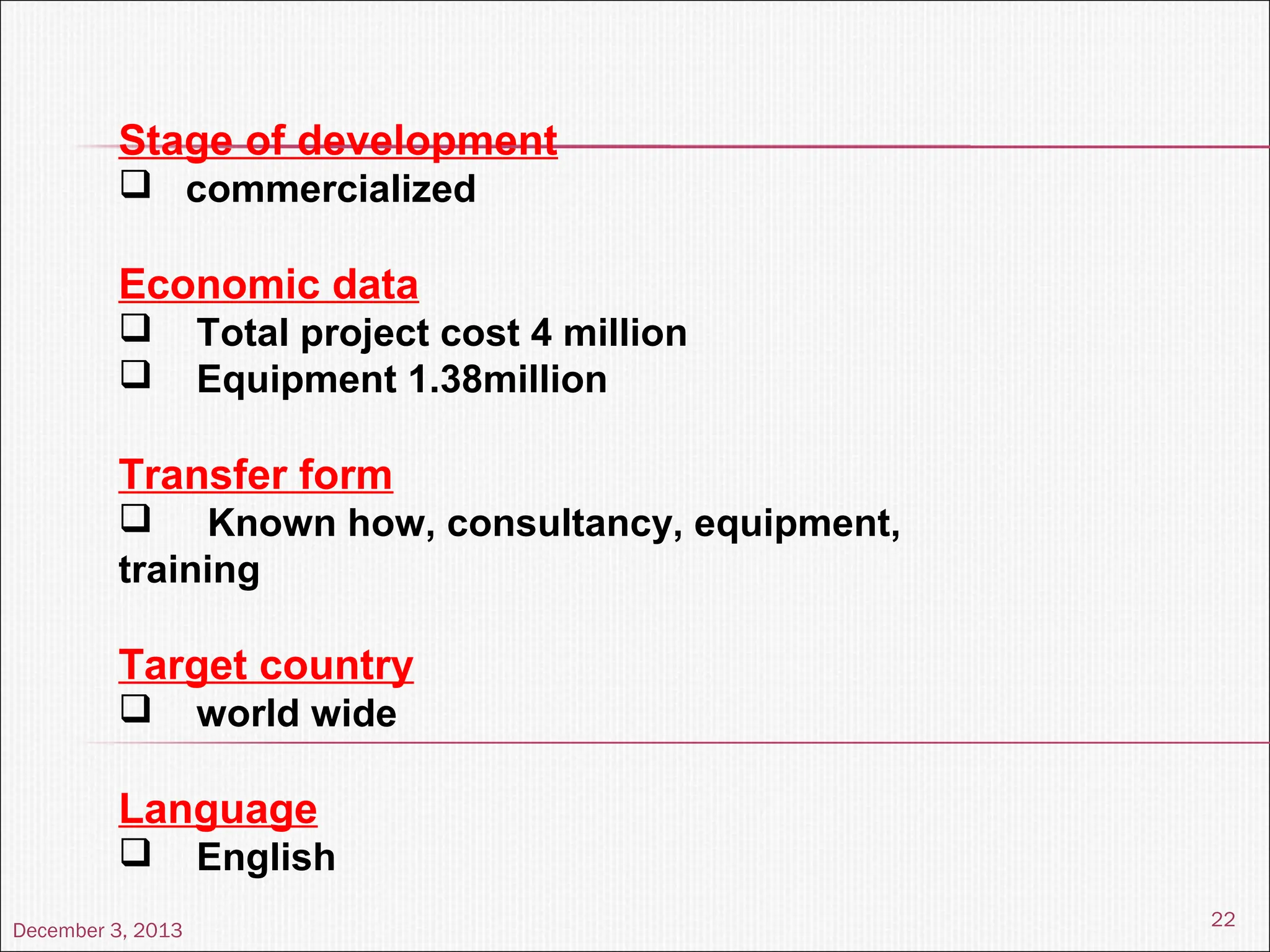
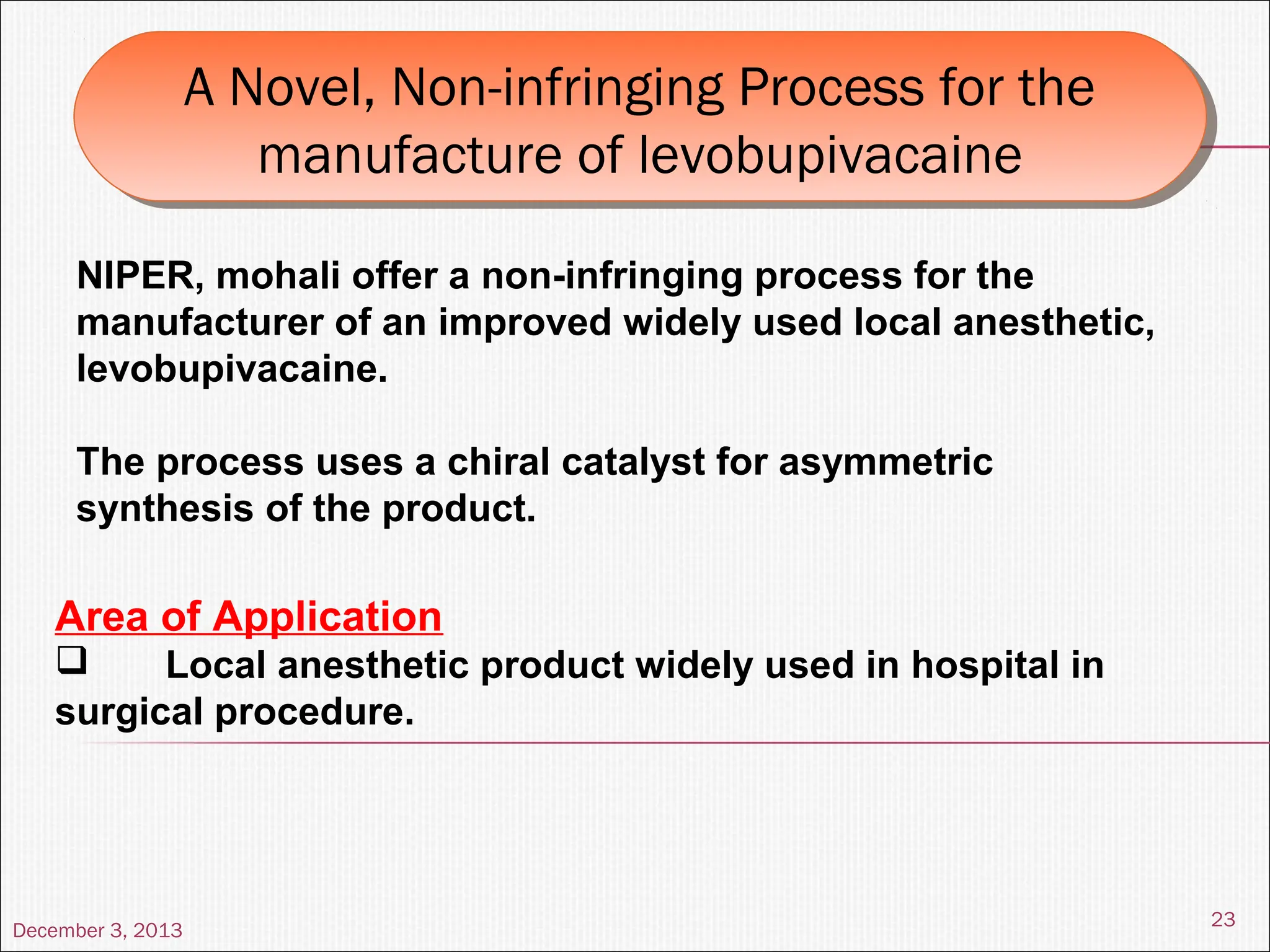
The document discusses the process and importance of technology transfer, focusing on transferring technology from research to production environments to meet public and private needs. It outlines various methods, agents, types of technology, and factors affecting the transfer process, as well as key roles and responsibilities of teams involved. Examples from real-world applications illustrate successful technology transfer in the pharmaceutical industry.