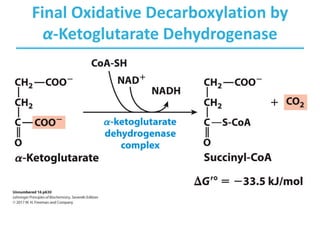
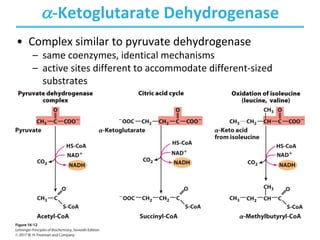
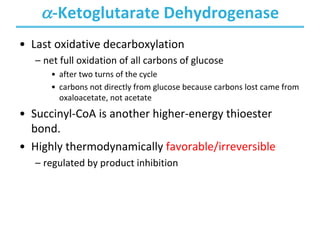
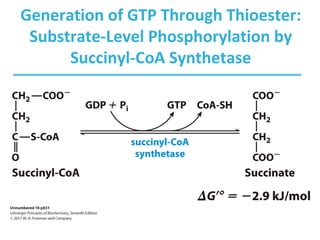
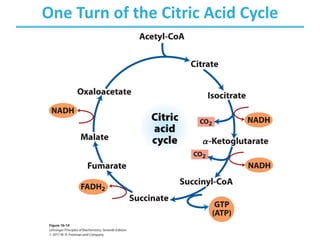
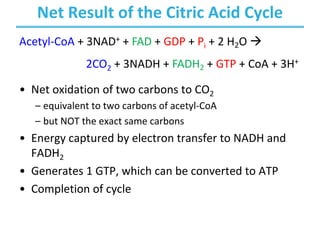
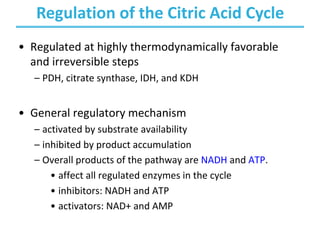
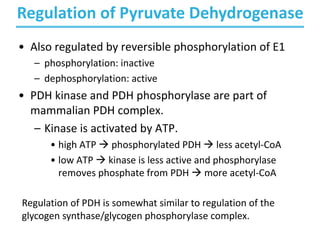
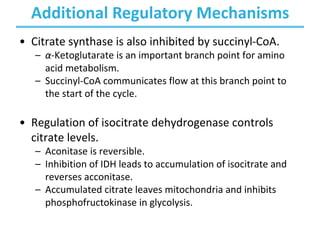
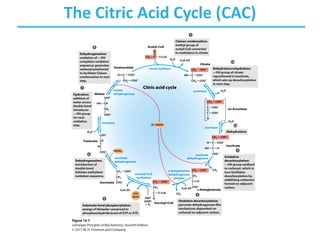
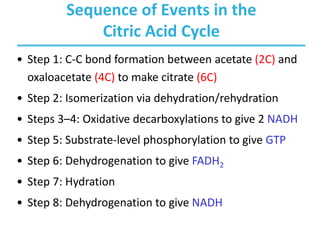
The citric acid cycle (TCA) is a series of oxidation-reduction reactions that harvests energy from acetyl groups and fully oxidizes them to carbon dioxide. It occurs in mitochondria and involves 8 steps where acetyl-CoA condenses with oxaloacetate to form citrate, followed by isomerization and oxidative decarboxylations to generate NADH and FADH2. One turn of the cycle fully oxidizes two carbons of acetyl-CoA to CO2 while capturing energy as electrons to power ATP synthesis. The cycle is regulated by substrate availability and product inhibition at key irreversible steps to optimize energy production.


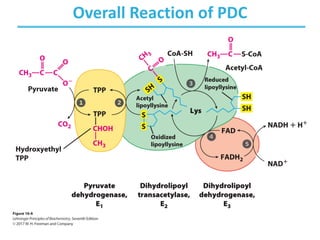

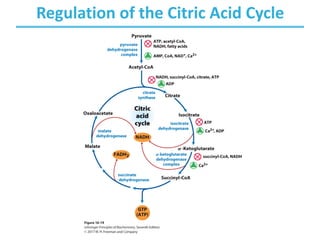
![Citrate Synthase
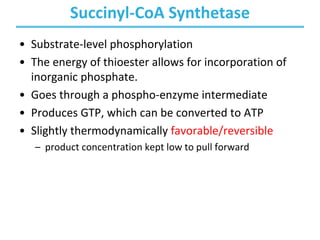
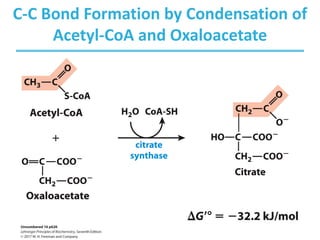
• Condensation of acetyl-CoA and oxaloacetate
• The only reaction with C-C bond formation
• Uses acid/base catalysis
– Carbonyl of oxaloacetate is a good electrophile.
– Methyl of acetyl-CoA is not a good nucleophile…
– …unless activated by deprotonation.
• Rate-limiting step of CAC
• Activity largely depends on [oxaloacetate].
• Highly thermodynamically favorable/irreversible
– regulated by substrate availability and product inhibition](https://image.slidesharecdn.com/tcacycle-230428054912-c09c6e85/85/TCA-Cycle-pdf-11-320.jpg)
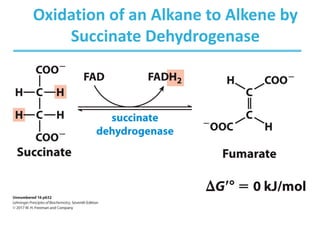
![Oxidative Decarboxylation by Isocitrate
Dehydrogenase
• Isozymes are specific for NADP+ (cytosolic) or NAD+ (mitochondrial).
• Decarboxylation releases CO2.
• Highly favorable/irreversible and regulated by [ATP]](https://image.slidesharecdn.com/tcacycle-230428054912-c09c6e85/85/TCA-Cycle-pdf-12-320.jpg)