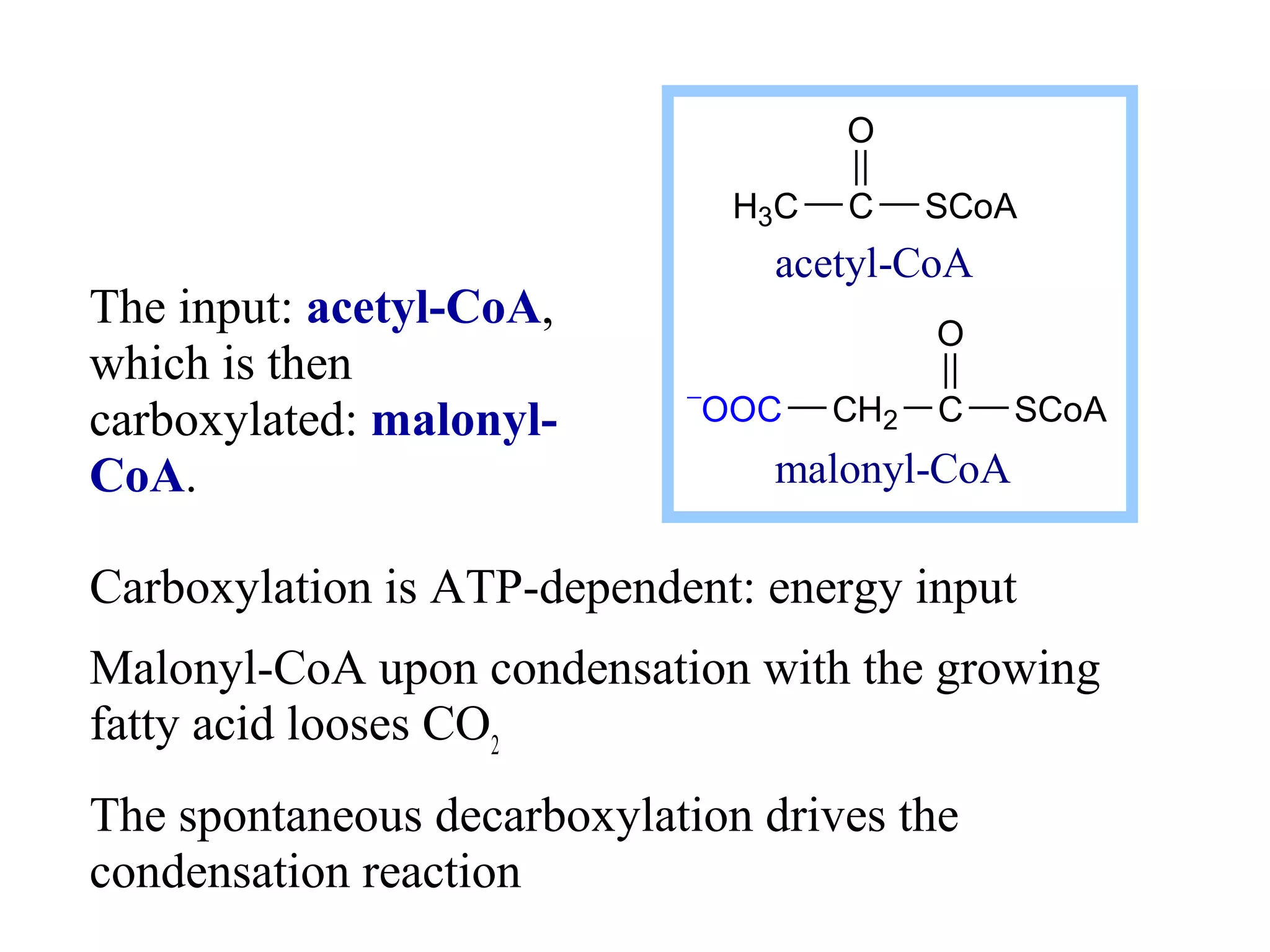
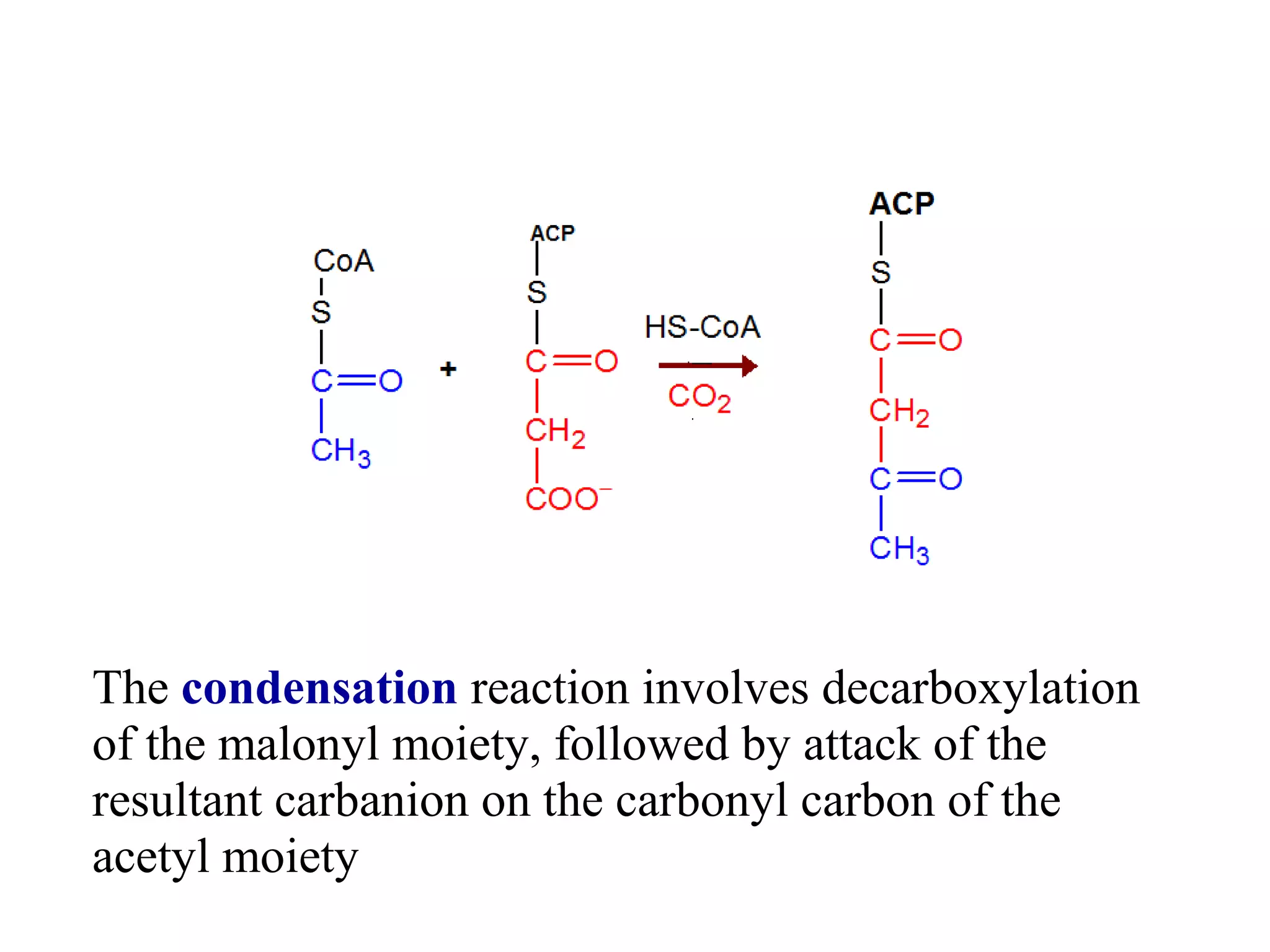
Fatty acid synthesis involves a two-step reaction where acetyl-CoA is carboxylated by biotin to form malonyl-CoA using ATP as an energy source. Malonyl-CoA then condenses with acetyl-CoA through a decarboxylation reaction to elongate the fatty acid chain. This cycle of condensation and decarboxylation is repeated until a 16-carbon fatty acid is produced, at which point a thioesterase domain catalyzes its release as palmitate. NADPH produced by the pentose phosphate pathway provides electrons to reduce substrates during chain elongation. Certain polyunsaturated fatty acids must be obtained through diet as mammals cannot introduce double bonds at specific