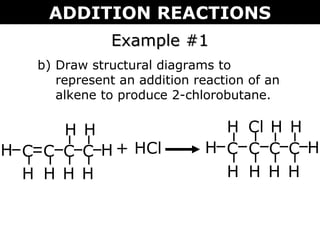
This document discusses addition reactions, which involve a double bond becoming a single bond by the addition of two groups of atoms. It describes several types of addition reactions: halogenation, hydrogenation, hydrohalogenation, and hydration. Halogenation and hydrohalogenation result in the formation of alkyl halides, while hydrogenation forms alkanes and hydration forms alcohols. The document provides examples of addition reactions and uses the Markovnikov rule to predict product formation.