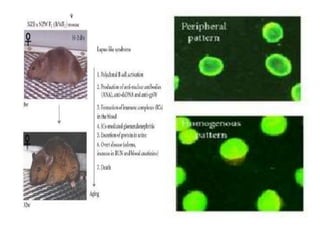
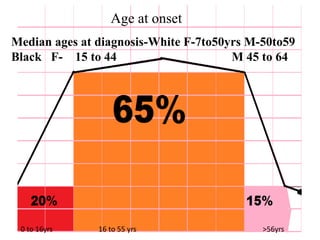
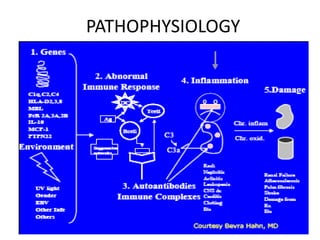
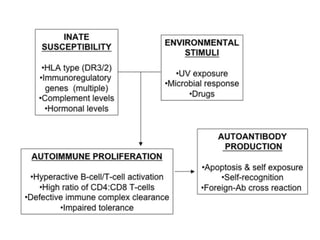
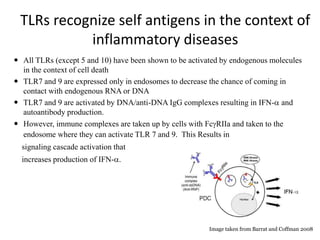
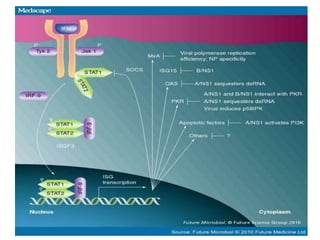
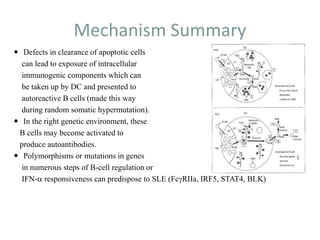
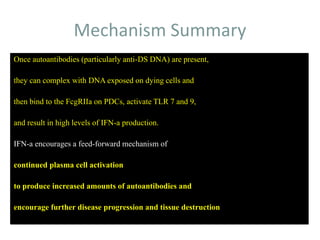
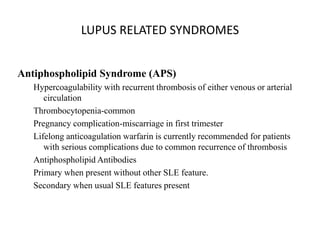
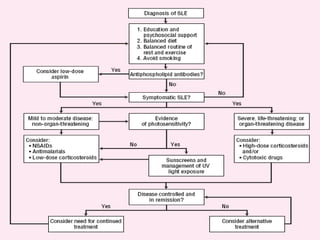
Systemic lupus erythematosus (SLE) is a complex autoimmune disorder characterized by the production of autoantibodies and immune complexes, influenced by genetic, environmental, and hormonal factors. Historically, lupus has evolved through various understandings of its manifestations, with significant advances in diagnosis and treatment occurring since the mid-20th century. Epidemiological data highlights disparities in incidence based on gender and ethnicity, with higher prevalence noted among certain populations, and various genetic, hormonal, and immunological factors contributing to its pathogenesis.























![Genetic factors confer highest hazard ratios of 5 to 25
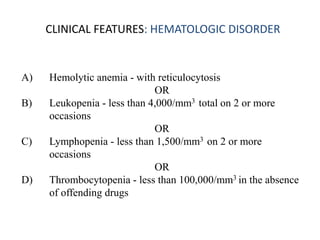
Deficiencies of complement - C1q (required to clear apoptotic cells) C 4A and B, C2
TREX1 gene mutation- (encodes 3 prime repair endonuclease1 that degrades DNA).
The most common genetic predisposition-MHC.
MHC - Genes for antigen presenting molecules
(class I -HLA-A,B, &-C and class II HLA molecules [HLA-DR, -DQ, & DP])
MHC also contains genes- complement components, cytokines,& heat shock protein.
Predisposing loci- DR2 &DR3, HR-2
But region is complex & involves multiple genes across the entire 120-gene region in
multiple ethnic groups](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-30-320.jpg)
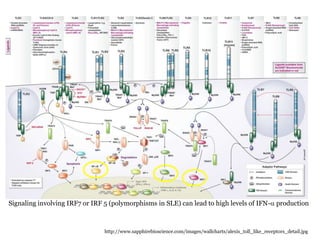
![Other genes
Innate immunity –
(IRF5, Stat4, IRAK1, TNFAIP3, SPP1), are associated with IFN alpha pathways
Overexpression of IFNa-induced genes is found- peripheral blood cells of 60% Lupus
Polymorphisms in STAT4, PTPN22&IRF5 -HR or increased sensitivity to IFN-a
Furthermore,
STAT4 and IRF5 may have additive effects genes involve lymphocyte signaling (PTPN22,
OX40L, PD-1, BANK-1, LYN, BLK)- activation or suppression of T/B cell
activation/survival
Clearance of immune complexes ( C1q, C4 &C2 , FcgammaRIIA, RIIA and RIIIB, CRP, and
integrin alpha M [ITGAM])
IL-10 is conferred by a variation in gene copy number rather than by different alleles eg, Fc
gamma R3&C4](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-31-320.jpg)

![Nephritis (2q34)
Hemolytic anemia (11q14)
Discoid lupus and thrombocytopenia (11p13)
Vitiligo (17p12)
production of certain autoantibodies (eg, anti-ds DNA [19p13.2])
Increased risk for end stage renal disease](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-34-320.jpg)







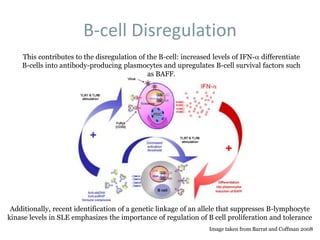

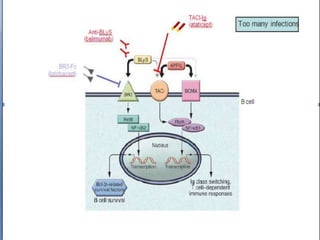
![[BAFF: A regulatory cytokine of B lymphocytes
involved in autoimmunity and lymphoid cancer].
[Article in Spanish]
Reyes S LI, León B F, Rozas V MF, González J P, Naves P R.
Source
Instituto de Ciencias, Clínica Alemana, Facultad de Medicina, Universidad del Desarrollo.
Abstract
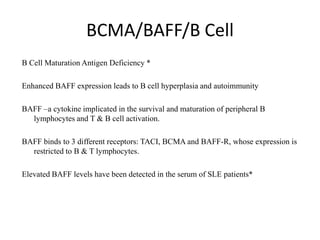
BAFF (B cell activating factor belonging to the TNF family) is a cytokine implicated in the survival
and maturation of peripheral B lymphocytes and T and B cell activation. BAFF binds to three
different receptors: TACI, BCMA and BAFF-R, whose expression is restricted to B and T
lymphocytes. BAFF and BAFF-R-deficient mice show a dramatic loss of peripheral B lymphocytes
and a severely reduced immune response. In contrast, an enhanced BAFF expression leads to B cell
hyperplasia and autoimmunity in mice. In vivo, administration of soluble decoy receptors for BAFF
effectively decreases disease progression in various autoimmune mouse models. These evidences
render BAFF as a potentially new therapeutic target. Elevated BAFF levels have been detected
in the serum of patients with autoimmune diseases, such as Systemic Lupus Erythematosus,
rheumatoid arthitis, Sjögren's syndrome, lymphoid cancers and HIV infection. In addition to
BAFF receptors, malignant B cells abnormally express BAFF, which attenuates apoptosis through
both autocrine and paracrine pathways. The data suggest that an increase in the expression of BAFF
induces an enhanced B and T cell activation and the survival of pathologically active B cells. In this
article, we review and discuss the participation of BAFF and its receptors in the immune response
and its involvement in immunodeficiency, autoimmunity, infections and lymphoid cancers as well as
the currently investigated therapies using BAFF antagonists in the treatment of these diseases.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-56-320.jpg)
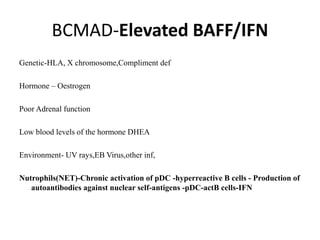


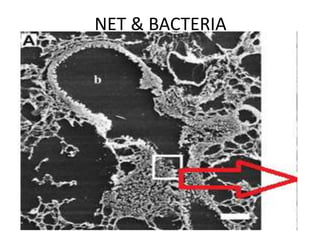
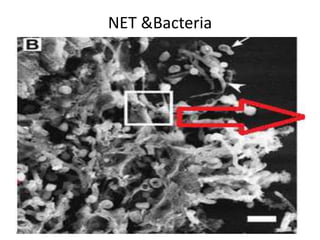
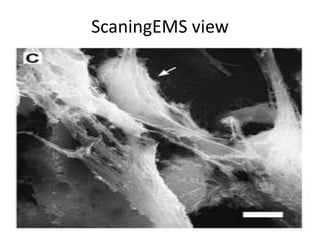
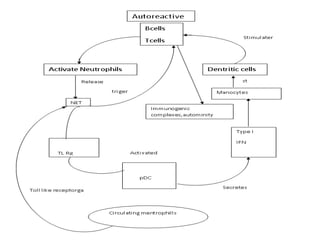
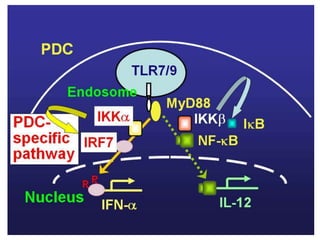
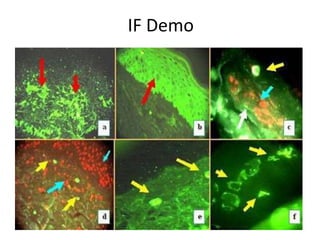








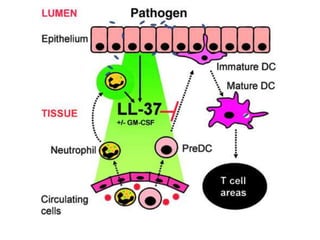
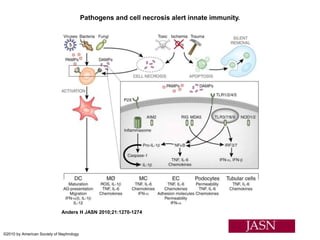
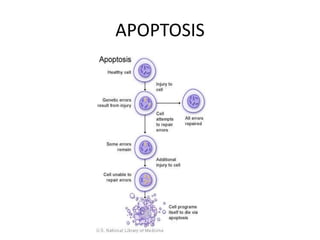
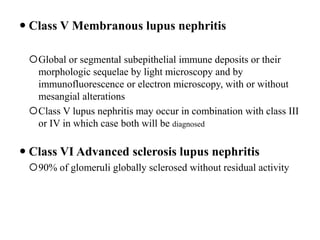
![Inflammasome-related sensors activate caspase 1, a necessary step for the secretion of
IL-1β.
Activation of such sensors has additional cell type–specific effects (e.g., in dendritic
cells [DC] or macrophages [MØ], mesangial cells [MC],35 glomerular endothelial
cells [EC],36 or podocytes
Cell necrosis can trigger identical effects because some intracellular molecules can act
as DAMPs on the same receptors
Apoptotic cell death and rapid clearance by phagocytes avoids unnecessary immune
activation.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-84-320.jpg)






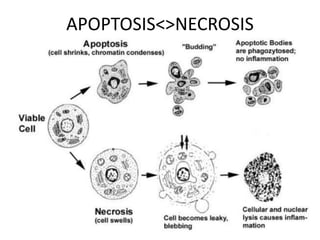
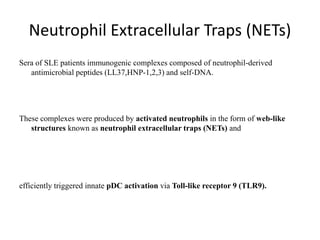
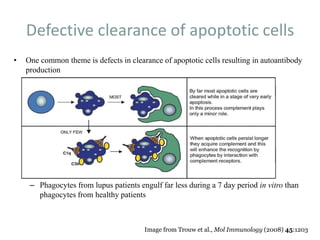
![APOPTOSIS
Similarly apoptosis the cell debris are
removed with out any
immunological reactions.
Ref:Hallmarks of the apoptotic and necrotic cell death process.(Pic)
Apoptosis includes cellular shrinking, chromatin condensation and margination at the nuclear
periphery with the eventual formation of membrane-bound apoptotic bodies that contain
organelles, cytosol and nuclear fragments and are phagocytosed without triggering
inflammatory processes.The necrotic cell swells, becomes leaky and finally is disrupted and
releases its contents into the surrounding tissue resulting in inflammation. Modified from [Van
Cruchten, 2002].](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-98-320.jpg)









![Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disorder
associated with a wide range of physical findings.
The risk of developing SLE is, at least in part, genetic, but it is a complex genetic
illness with no clear mendelian pattern of inheritance. The disease tends to occur in
families.
Siblings of SLE patients have a risk of disease of about 2%.
However, even identical twins with SLE are concordant for disease in only 25% of
cases and are therefore discordant (ie, where one twin has SLE and one does not) in
about 75% of cases.[1]
MHC on chromosome 6, which contains the human lymphocyte antigens (HLA), was
the first described genetic link to SLE.
The protein products of the HLA genes are critical components of cell-to-cell
communication in the immune system.
Indeed, in some cases, HLA genes are more highly related to lupus-associated
autoantibodies than to the disease itself.
Nonetheless, carriage of specific alleles of HLA imparts about a 2-fold risk of SLE
above the general population.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-121-320.jpg)
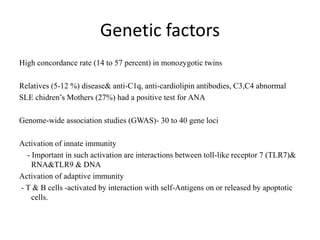
![Genome-wide genetic association studies (GWAS) have been performed in large
collections of SLE patients and controls.
These genome-wide studies of up to 500,000 single-nucleotide polymorphisms (SNPs)
have identified at least 30 and perhaps up to 50 genetic associations for SLE,[6, 7]
and replication studies have confirmed these findings, in nonwhite as well as white
cohorts
However, only a fraction of the genetic risk for SLE has so far been identified. Rare
alleles and mutations that impart a moderate risk of SLE remain undiscovered and
cannot be found by GWAS. Gene-gene interaction is virtually unexplored.
Nevertheless, although few of GWAS have identified actual causative alleles that
impart risk of SLE, the findings do have common themes.
Many of the genes implicated thus far can be categorized as involved in B lymphocyte
activation, apoptosis, or the interferon signaling pathway.
Such insight into the genetic pathogenesis of SLE may suggest new therapeutic targets
for the disease down the road.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-122-320.jpg)










![Clinical Implications
Although SLE is generally a complex genetic illness, there are several examples of
mutations that can produce a monogenetic form of the illness
Complete deficiency of the early complement components C2, C4, and C1q results in
SLE in 75%, 10%, and 90% of cases, respectively
However, complete complement deficiencies are quite rare and account for only a tiny
percentage of SLE cases.[3] More commonly, a low gene copy number of C4 is seen
as a risk factor for SLE, whereas a high copy number of C4 is protective against
SLE.[4]
Sex-chromosome copy number variations are also implicated in the risk of SLE. SLE is
about 10 times more common in women than in men. However, men with SLE have
15 times the risk of Klinefelter syndrome (47,XXY) as compared with the average
population, and the risk of SLE among men with 47,XXY is equal to that of
women.[5]
These data suggest that the predisposition of women to developing SLE is related to X
chromosome copy number, not to sex.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-134-320.jpg)






























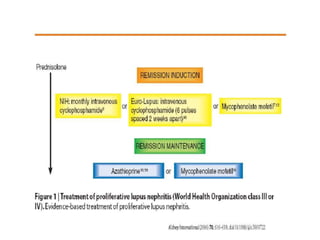
![In 1984 a pooled analysis of eight studies of lupus nephritis, comprising 250 patients
(including children), 198 renal biopsies and 167 patients with biopsy evidence of diffuse
proliferative lupus nephritis, was published
Three of the studies came from the National Institutes of Health (NIH), and the study by
Donadio discussed above was also included.
Of the 250 patients, 113 received only corticosteroids, and the rest received corticosteroid
and other immunosuppressive agents (azathioprine and CTX)
Patients receiving the corticosteroid and another agent had a lower rate of deterioration of
kidney function.
The New England Journal of Medicine [4].](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-180-320.jpg)

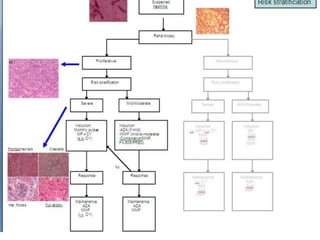
![None of these studies was a head-to-head comparison
of the two immunosuppressive agents
However, the pooled analysis added credence to Donadio's finding that prednisone in
combination with another immunosuppressive drug was more efficacious than
prednisone alone.
Within the limitations of this kind of analysis, azathioprine appeared to be a helpful
drug in the management of diffuse proliferative lupus nephritis without the risk of
increasing non-renal (?infective) deaths, as was suggested with CTX
The latter part of the 1980s was dominated by a series of publications from the National
Institutes of Health on the interim and final outcomes of different treatment
protocols for the treatment of lupus nephritis
Another follow-up report just 1 year later, focusing on histologic predictors of outcome,
was published in The New England Journal of Medicine in 1984 and did not find a
difference among the different cytotoxic-drug regimens and renal outcome].](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-182-320.jpg)



![It was new therapy, carried the cache of the National Institutes of Health and was the
protocol to which all others were compared thereafter
Furthermore, as Lewis observed: ‘The tendency to recommend parenteral
cyclophosphamide may in part reflect the mystique associated with a more invasive
intervention’
Finally, despite evidence that started to accrue suggesting that this therapy may not
necessarily lead to superior results compared to other immunosuppressive
regimens, it continued to be defended by the original investigators].](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-186-320.jpg)

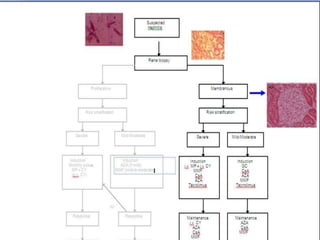
![The best thing about MMF
• is that it will convince people that there are therapies for lupus nephritis other
than pulse CTX
• The issue of induction of therapy was re-addressed by the study of Ginzler and
colleagues where patients were randomized to receive MMF versus pulse CTX and
was designed as a short-term (24 week) equivalency study
• The pregnant patient with lupus represents a special challenge. Azathioprine is a D
class drug, acknowledging that there is evidence of human fetal risk, but the
benefits from its use may be acceptable in the pregnant patient with active lupus
• This is extrapolated from the pregnant transplant patient where this drug is usually
not discontinued [16].](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-188-320.jpg)






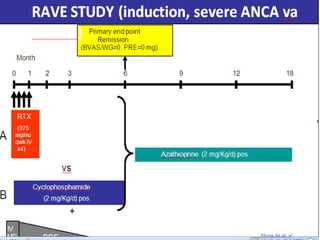
![• Background:
• The 36-month maintenance phase of the ALMS study (NCT00377637) compared
the efficacy and safety of mycophenolate mofetil (MMF) with azathioprine (AZA)
in patients with lupus nephritis (LN) classes III, IV and V achieving a clinical
response in the induction phase with corticosteroids (CS) and either MMF or
cyclophosphamide (IVC).
• Methods:
• Patients were re-randomized 1:1 to a double-blind comparison of either placebo
plus either oral MMF (2 g/day) or oral AZA (2 mg/kg/day). Patients were permitted
to receive corticosteroids (maximum dose: 10 mg/day prednisone or equivalent).
The primary efficacy outcome measure was time to treatment failure (death, end-
stage renal disease, sustained doubling of serum creatinine, and/or renal flare
[proteinuric or nephritic]). Patients who withdrew before reaching the primary
endpoint were censored at the time of withdrawal. Although this was primarily an
LN population, substantial extra-renal assessments were performed. Extra-renal
secondary parameters included time to major extra-renal flare (British Isles Lupus
Assessment Group [BILAG] score category A in one extra-renal system or three
systems with concurrent category B scores) and the characterization of extra-renal
activity. Immunology secondary parameters (levels of complement proteins C3 and
C4, and titers of antibodies to double-stranded DNA [anti-dsDNA]) and adverse
events (AEs) were also assessed.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-195-320.jpg)
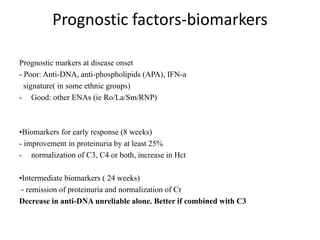
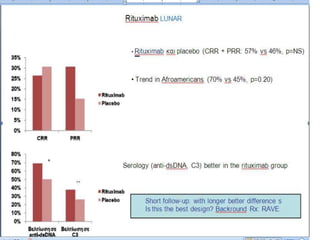
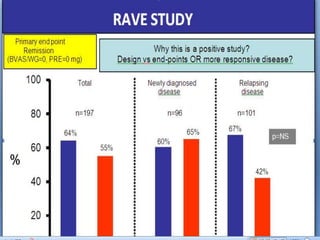
![Results
• Of 227 patients randomized (intent-to-treat population), 127 completed the full 3
years (MMF, 73/116 [62.9%]; AZA, 54/111 [48.6%]): MMF was superior to AZA
in the primary endpoint (p=0.003). Extra-renal disease characteristics and
immunology parameters were similar across groups at baseline. There were very
few occurrences of major extra-renal flare in either group during the study (8
[6.9%] for MMF, 7 [6.3%] for AZA), and time to major extra-renal flare did not
differ between groups (p=0.936). However, there were differences in the
characteristics of extra-renal activity. The most common manifestation of major
extra-renal flare in the MMF group was mucocutaneous and in the AZA group was
hematological. In the MMF group, 75 subjects (65.2%) experienced lupus-related
AEs compared with 79 (71.2%) in the AZA group, with musculoskeletal events
being the most common in both groups (MMF, 39/115 [33.9%]; AZA, 37/111
[33.3%]). At the end of the study, in patients who had completed 3 years, mean C3
and C4 levels were lower in the AZA group and mean anti-dsDNA titers were lower
in the MMF group; differences were not statistically significant.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-196-320.jpg)

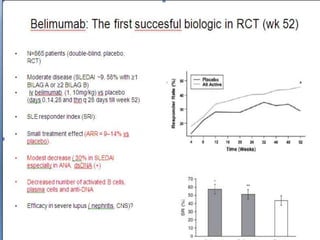
![The use of nontargeted agents:
Important recent studies
The overwhelming majority of agents in development are biologics. However, some
nonbiological agents and drugs that are on the market for other disorders have been
or are under study for SLE. A detailed discussion of these studies is beyond the
scope of this minireview, but the salient points are summarized below:
1. Fish oil is ameliorative in patients with mild activity [6].
2. A large trial evaluating the efficacy of vitamin D is in progress (NCT 00418507).
3. The Canadian Cooperative Consortium recently demonstrated that methotrexate is
steroid sparing and has anti-inflammatory properties [7].
4. Mycophenolate mofetil is equivalent to cyclophosphamide as induction therapy for
SLE nephritis and is superior to azathioprine for maintenance [8,9].
5. Topical pinecrolimus and tacrolimus are effective for chronic cutaneous SLE [10].
6. Leflunomide improves SLE arthritis [11].
7. Dehydroepiandrostrone has modest effects at best in mild SLE and may diminish
fatigue and bone demineralization, as well as having steroid sparing properties [12].](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-198-320.jpg)


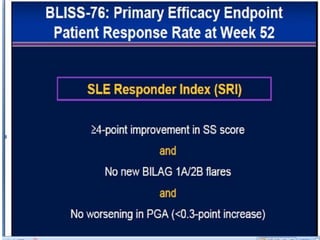
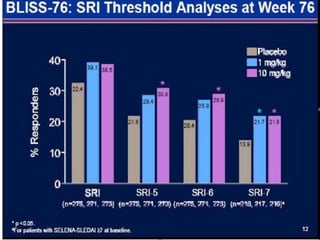
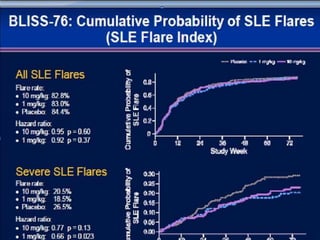
![Ground rules: Requirements for a new
SLE drug
• The June 2010 FDA guidelines indicate that a candidate SLE drug should meet its
primary endpoint in two adequate well-controlled trials demonstrating superiority [4].
Studies should be at least 1 year in duration, and enrollees should fulfill the American
College of Rheumatology criteria for SLE. Steroid use variability should be minimized,
and sparing effects, if any, should be defined. Study patients should be stratified by the
severity of their SLE, with the British Isles Lupus Assessment Group (BILAG) 2004 [5]
guidelines being the preferred index for measuring disease reduction (although the
Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), European
Community Lupus Activity Measure (ECLAM) and Systemic Lupus Activity Measure
(SLAM) are also acceptable). The document provides definitions for partial clinical
response, remission, reduction in flare and increase in time to flare; encourages the use
of patient-reported outcome measures; and leaves the door open for biomarkers and
surrogate markers (none of the current ones being acceptable) potentially applicable to
shorten the duration of a trial as well as improving our measurement of disease activity.
Any agent must demonstrate a satisfactory safety profile, and the document supports the
use of organ-specific measures (for example, the Cutaneous Lupus Activity Disease Area
and Severity Index (CLASI) for cutaneous disease), especially if the drug is efficacious
for one aspect of the disease but not another. The 2010 guidance document takes into
account "lessons learned" and nuances that make SLE drug development so complex.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-202-320.jpg)


![Anti-inflammatory targets:
Anti-TNF, cytokines, toleragens and cell surface receptor inhibition
• Many patients with RA who have SLE overlap disease have been treated with anti
tumor necrosis factor products [23]. Only infliximab has been studied to any extent
in pure SLE. Synovitis can be helped, but extra-articular manifestations may
worsen and anti-DNA, anticardiolipin levels can appear or increase.
• Anakinra (anti-IL-1Ra) is not effective for SLE, but tociluzumab (an anti-IL6) was
quite potent in a 16-patient open label phase I trial at the National Institutes of
Health [24]. An anti-interleukin (IL)-6 (CNTO 136; Johnson & Johnson, New
Brunswick, NJ, USA/Centocor, Horsham, PA, USA) nephritis trial is due to start in
late 2010. IL-10 can have favorable or unfavorable effects in SLE because of its
pleomorphic properties; however, a favorable phase I safety trial of an anti-IL-10
(Schering, Berlin, Germany) is not likely to lead to further development because of
its numerous contradictory actions. Promising strategies in murine SLE include
inhibition of IL-12, -17, -18, -21 and -23, which may have translatable effects in
humans.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-206-320.jpg)
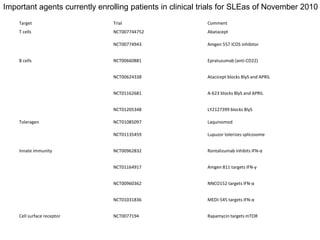
![• La Jolla Pharmaceutics (La Jolla, CA, USA) LJP394 (Riquent) was an anti-anti-
DNA B cell toleragen and edratide (TEVA, Petach Tikva, Isreal) a toleragen to the
anti-16/6 anti-DNA idiotype [25,26]. Both were safe in trials involving hundreds of
patients, but neither was effective enough to warrant further investigation.
Laquinomod ( TEVA) has been tested in over 3,000 patients with multiple sclerosis
and inflammatory bowel disease and appears to shift Th1 to Th2. An arthritis and
nephritis trial was begun in late 2010. Lupuzor (Cephalon, Frazer, PA, USA) is a
splicosomal peptide with U1 snRNP that promotes tolerance by preventing the
proliferation of CD4+ T cells, as well as promoting secretion of IL-10 and
decreasing anti-DNA in a European study. A phase IIb study is in progress.
• Syk kinase inhibits intracellular kinases, and its clinical efficacy was demonstrated
with R788 (Rigel, South San Francisco, CA, USA) in phase III RA trials [27].
There are plans to study this agent in SLE. Sirolimus (rapamycin) binds the
regulatory kinase mTOR and is used for renal transplant rejection prevention. Many
SLE patients with transplants currently take this agent, and a phase II trial is in
progress.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-207-320.jpg)
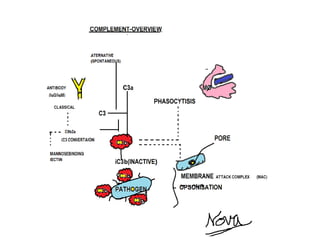
![Innate immunity
including complement
Monoclonal antibodies to C5a were studied and shown to be safe in a phase I trial a
decade ago with eculizumab (Solaris/Alexion, Cheshire, CT, USA),
an agent now available for paroxysmal nocturnal hemoglobinuria [28].
A newer preparation from Novo Nordisk (Novo Nordisk, Bagsvaard, Denmark). had its
SLE trial halted due to concerns relating to neutropenia in control patients.](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-208-320.jpg)
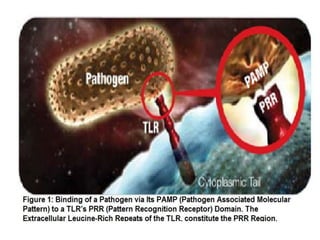
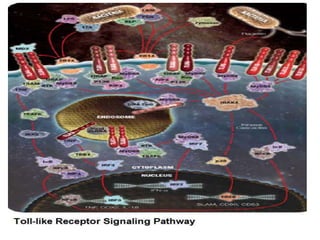
![• Toll-like receptors (TLRs)-7 and -9 in immature dendritic cells are activated by
complexes of self-protein and RNA or DNA. These complexes are normally rapidly
cleared but accumulate in SLE because of clearance defects. TLR-7 and -9
activation induces secretion of interferons and promotes inflammation. Antimalarial
drugs target TLR-7 and -9, and the development of a small oral molecule with
similar actions has generated great interest from several companies (for example,
ESAI (Woodcliff Lake, NJ, USA), Coley (Dusseldorff, Germany)/Xiphon (New
Castle, Delaware, USA)/Pfizer (New York, NY, USA)). Medi-545 (sifalimumab;
Medimmune (Gaithersburg, MDm USA)/Astra Zeneca (Wilmington, DE, USA))
and rontalizumab (Roche) can decrease the α-interferon signature within days by
90% by looking at protein and gene expression and clear lesions in serial skin
biopsies in phase I studies [29,30] (Table (Table2).2). They are currently in phase
III trials. NNC0152 (Novo Nordisk) and Neovasc are further behind in
development, as is an agent which targets γ-interferon (AMG 811; Amgen).](https://image.slidesharecdn.com/slesympfnal29nov11-181118092514/85/SYSTEMIC-LUPUS-ERYTHEMATOSIS-209-320.jpg)