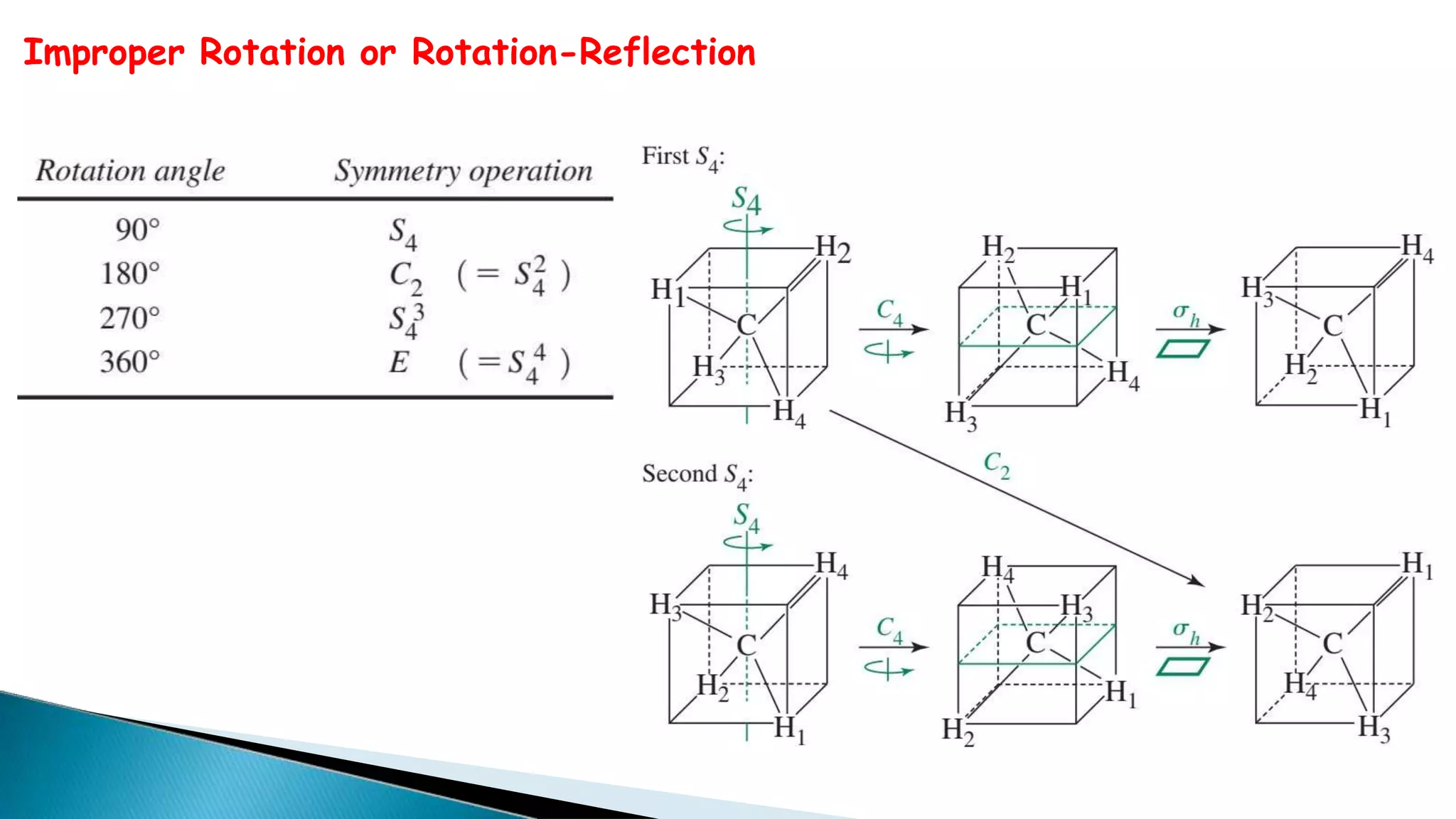
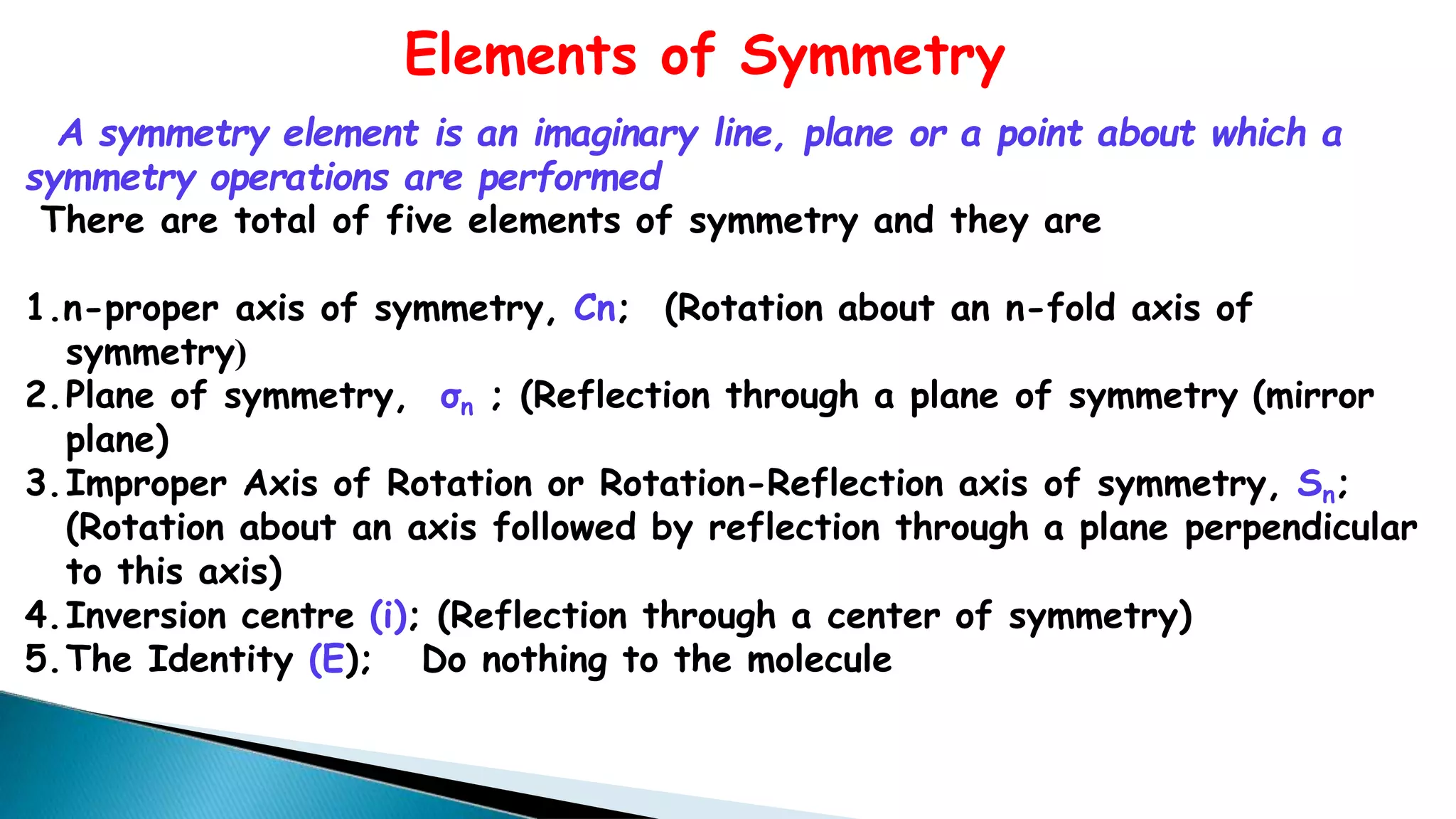
1) A symmetry operation leaves an object in an indistinguishable configuration from the original. There are five symmetry elements - n-fold rotation axis (Cn), plane of symmetry (σn), improper rotation axis (Sn), inversion center (i), and identity (E).
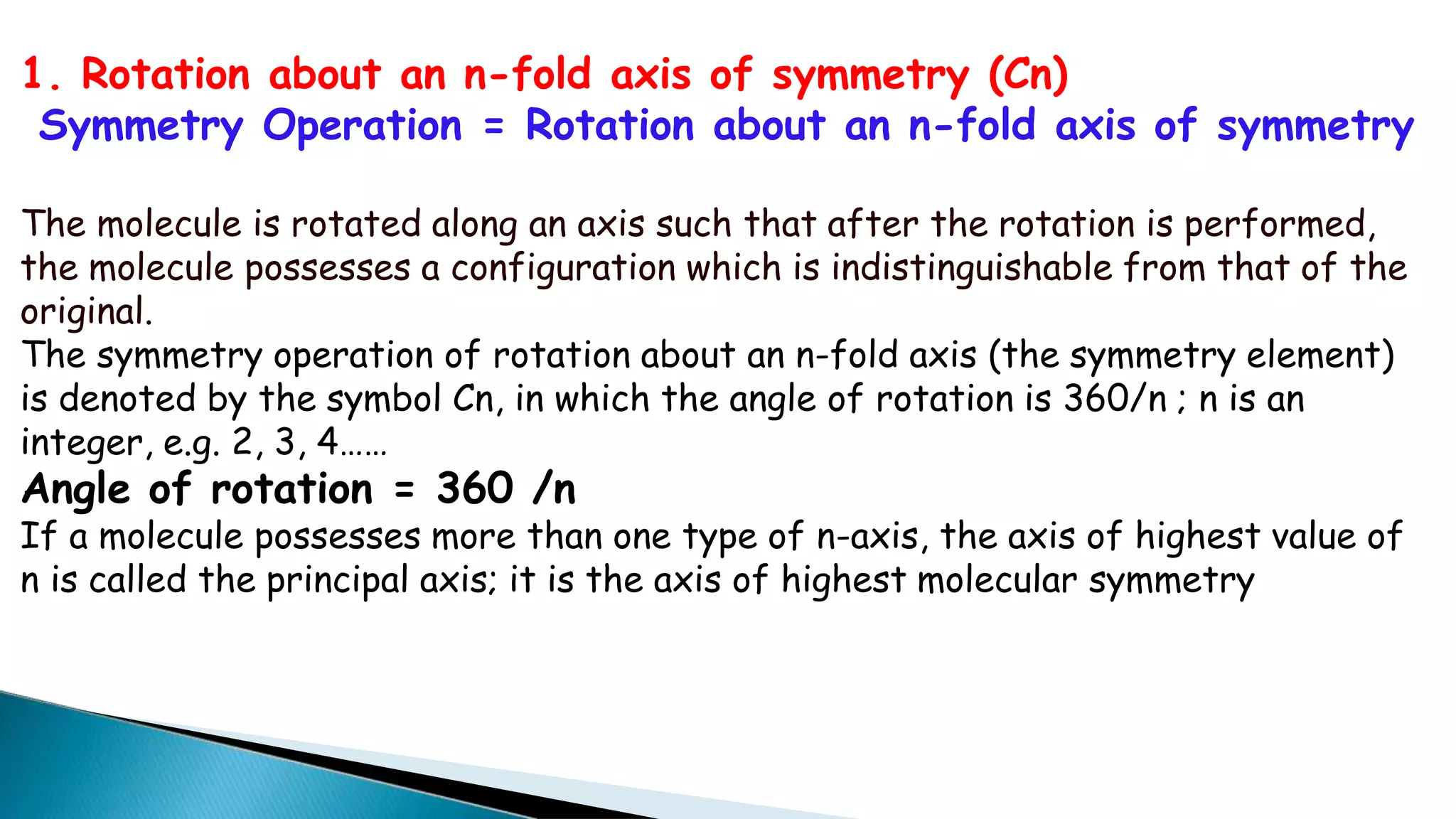
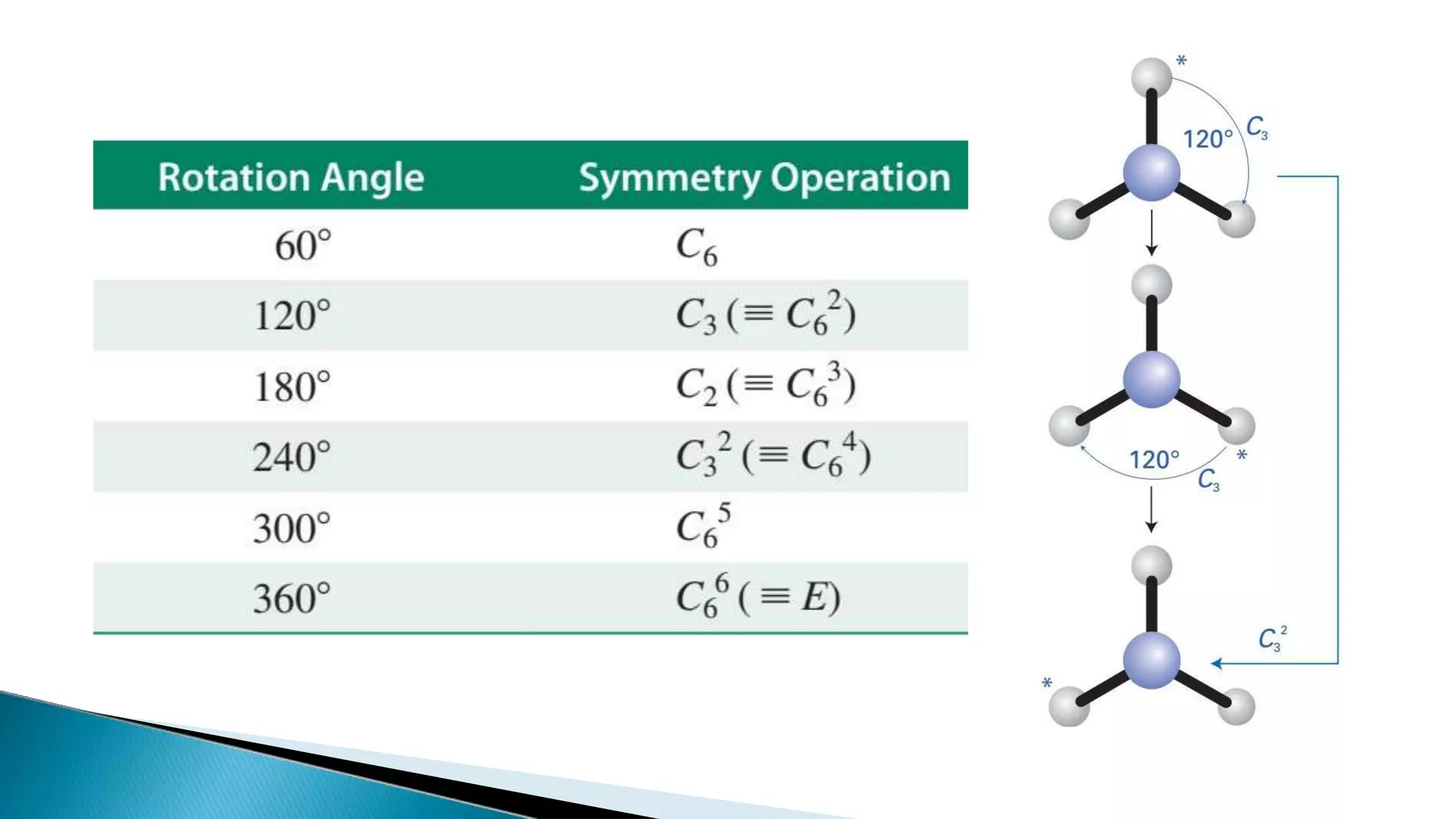
2) Cn rotation involves rotating around an axis by 360/n degrees. σn reflection involves reflecting through a plane. Sn rotation-reflection involves rotating and then reflecting. Inversion (i) involves reflecting through the center.
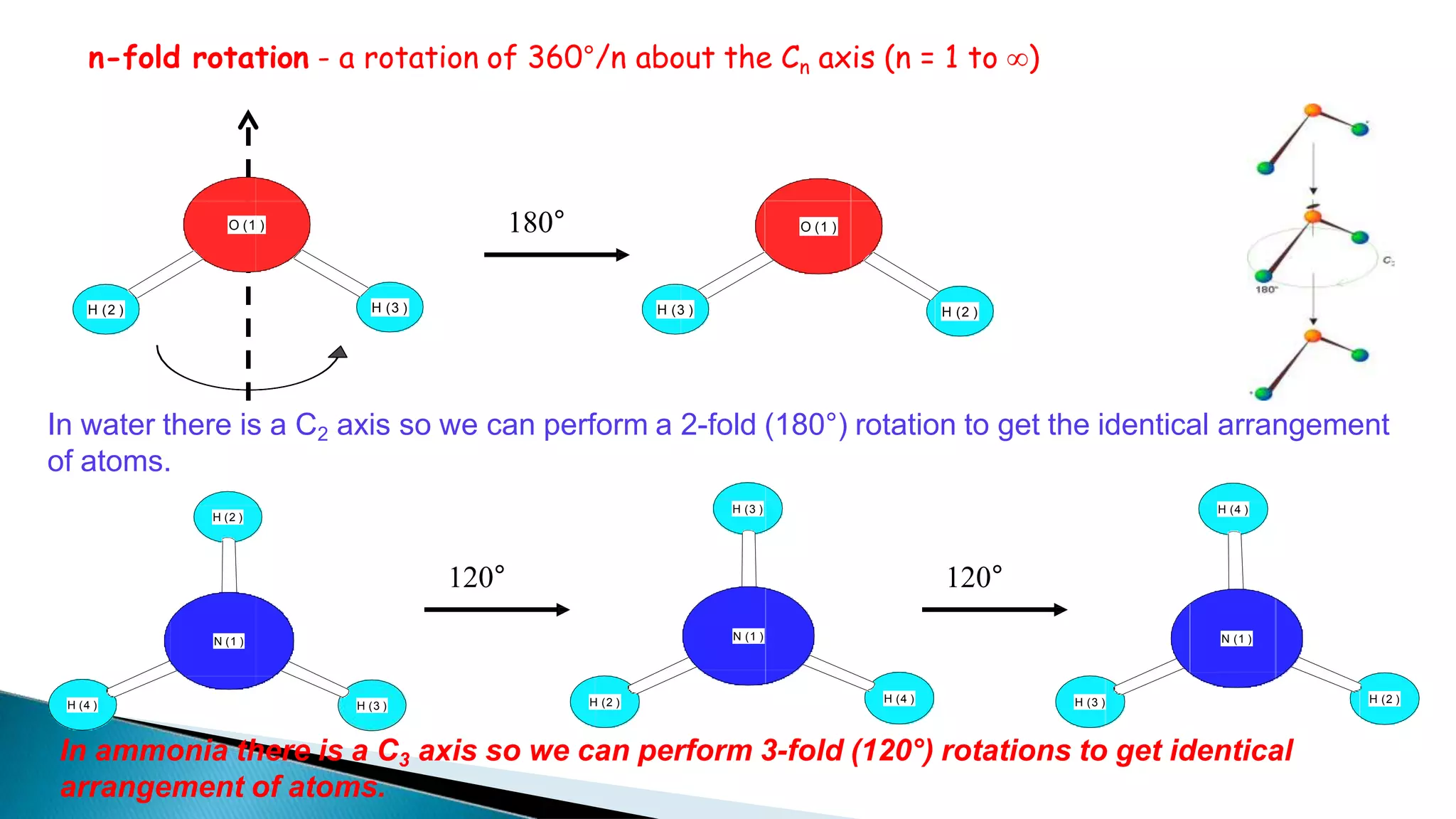
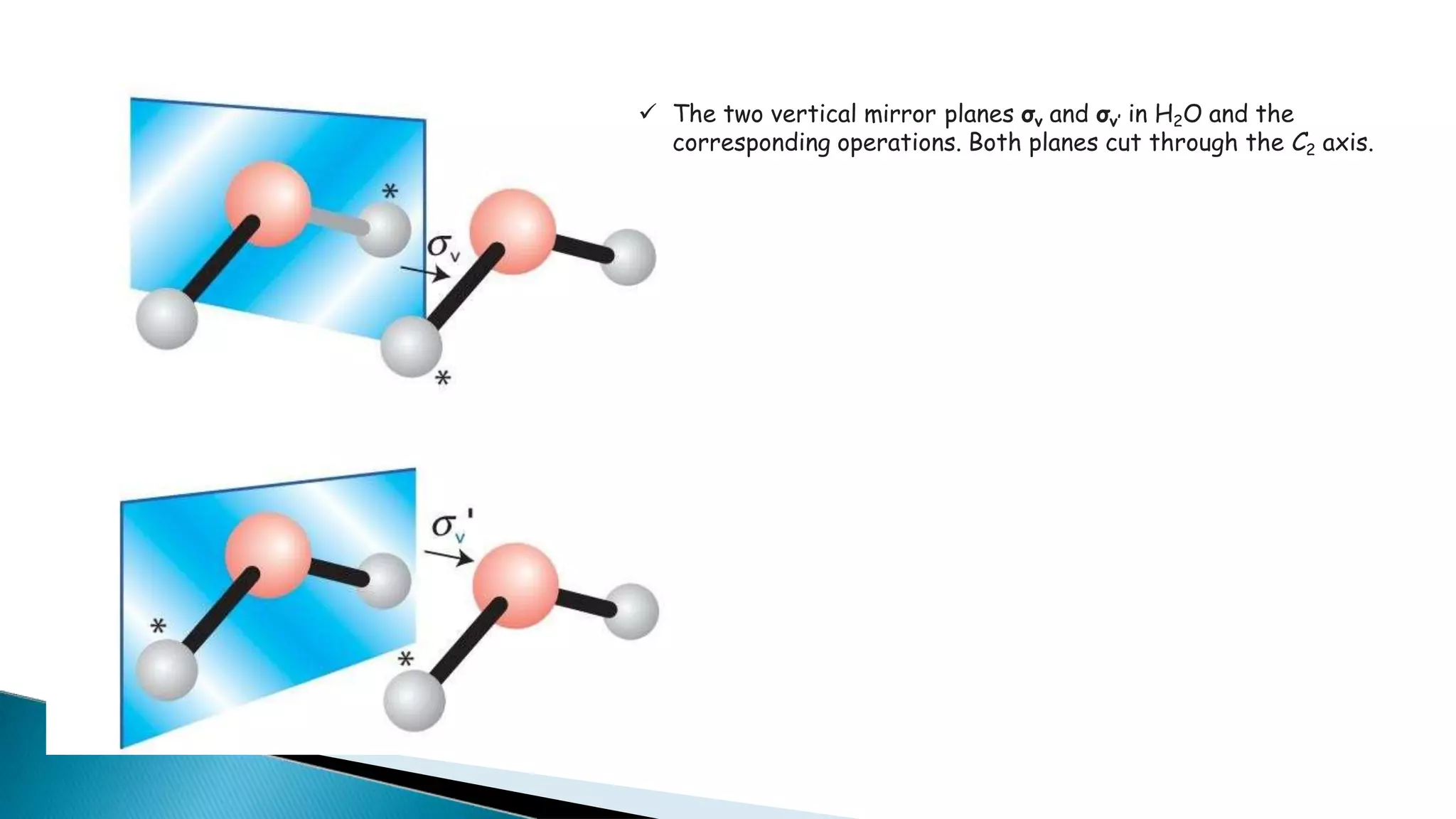
3) Common molecules like water (C2), ammonia (C3), methane (C3v) demonstrate different symmetry elements and operations



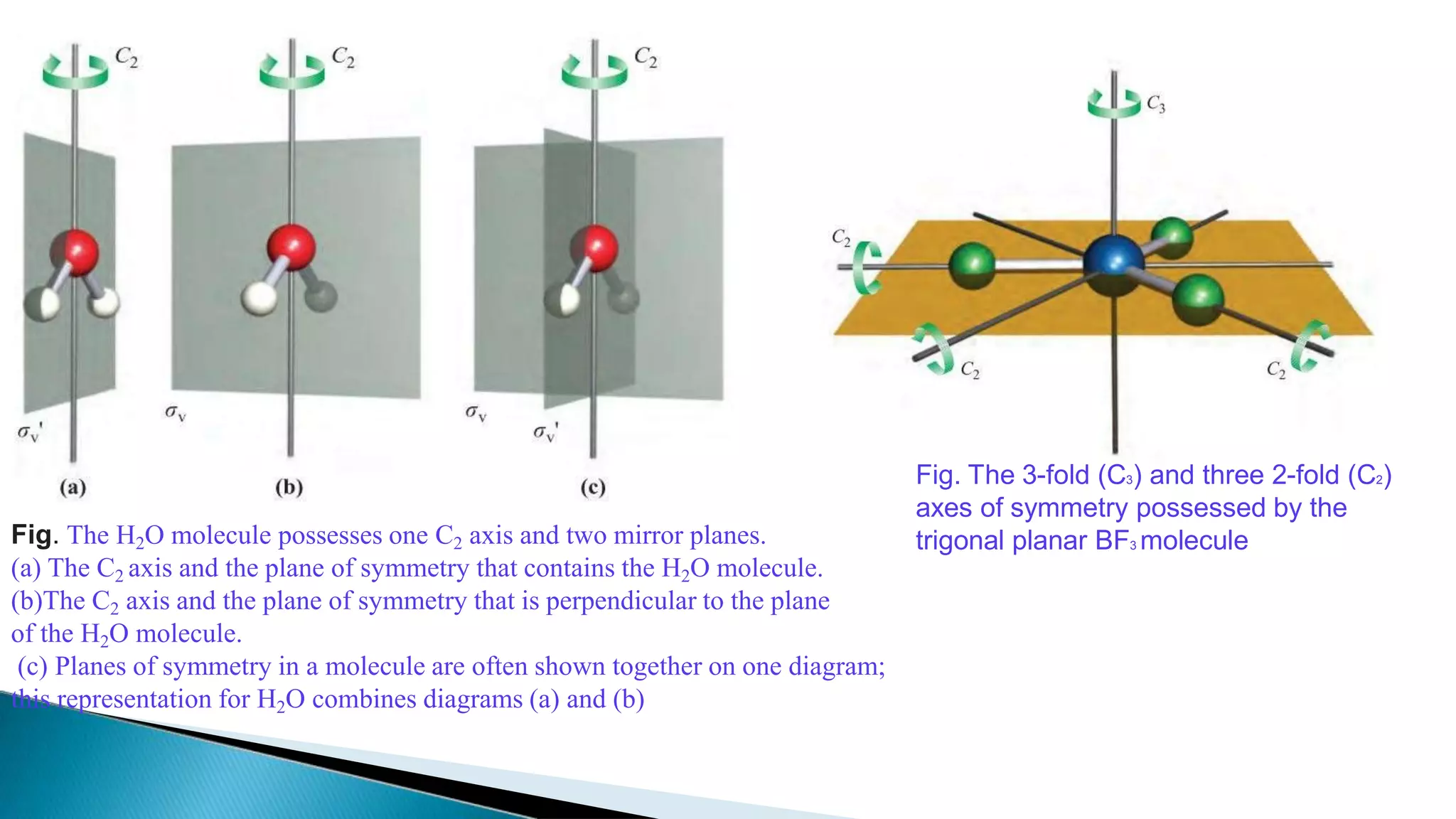
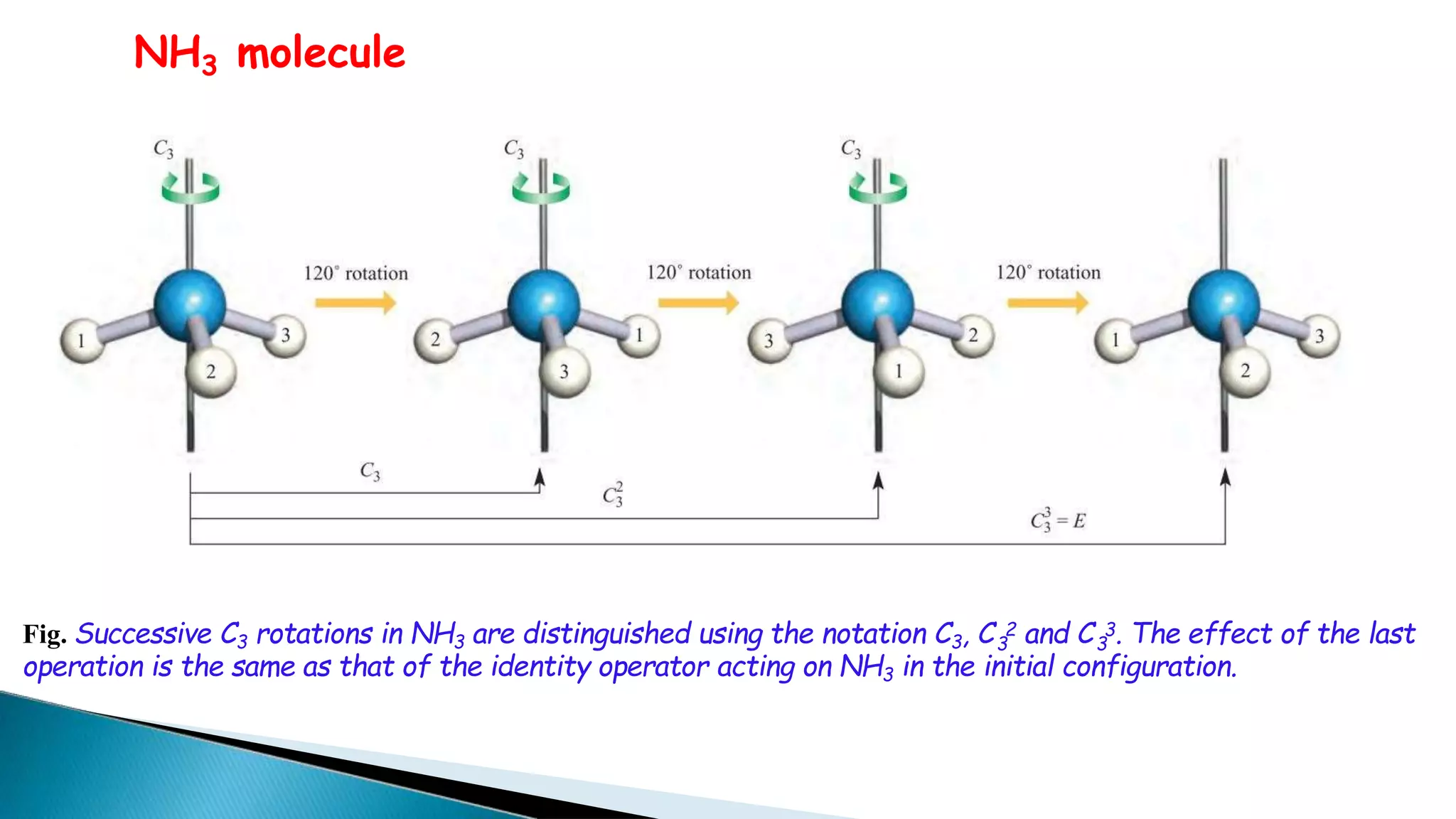



![3. Inversion centre (i)
Symmetry Operation = Reflection through a centre of symmetry
If reflection of all parts of a molecule through the centre of the molecule produces an
indistinguishable configuration, the centre is called a ‘centre of symmetry’,
It also called a centre of inversion and id designated by the symbol i.
Each of the molecules CO2 , trans-N2F2 , SF6 and benzene possesses a center of symmetry.
Each point moves through the center of the molecule to a position opposite the original
position and as far from the central point as where it started.
[x, y, z]
i
[-x, -y, -z]](https://image.slidesharecdn.com/999970921-221221102531-8ac9cd44/75/99997092-1-pptx-14-2048.jpg)