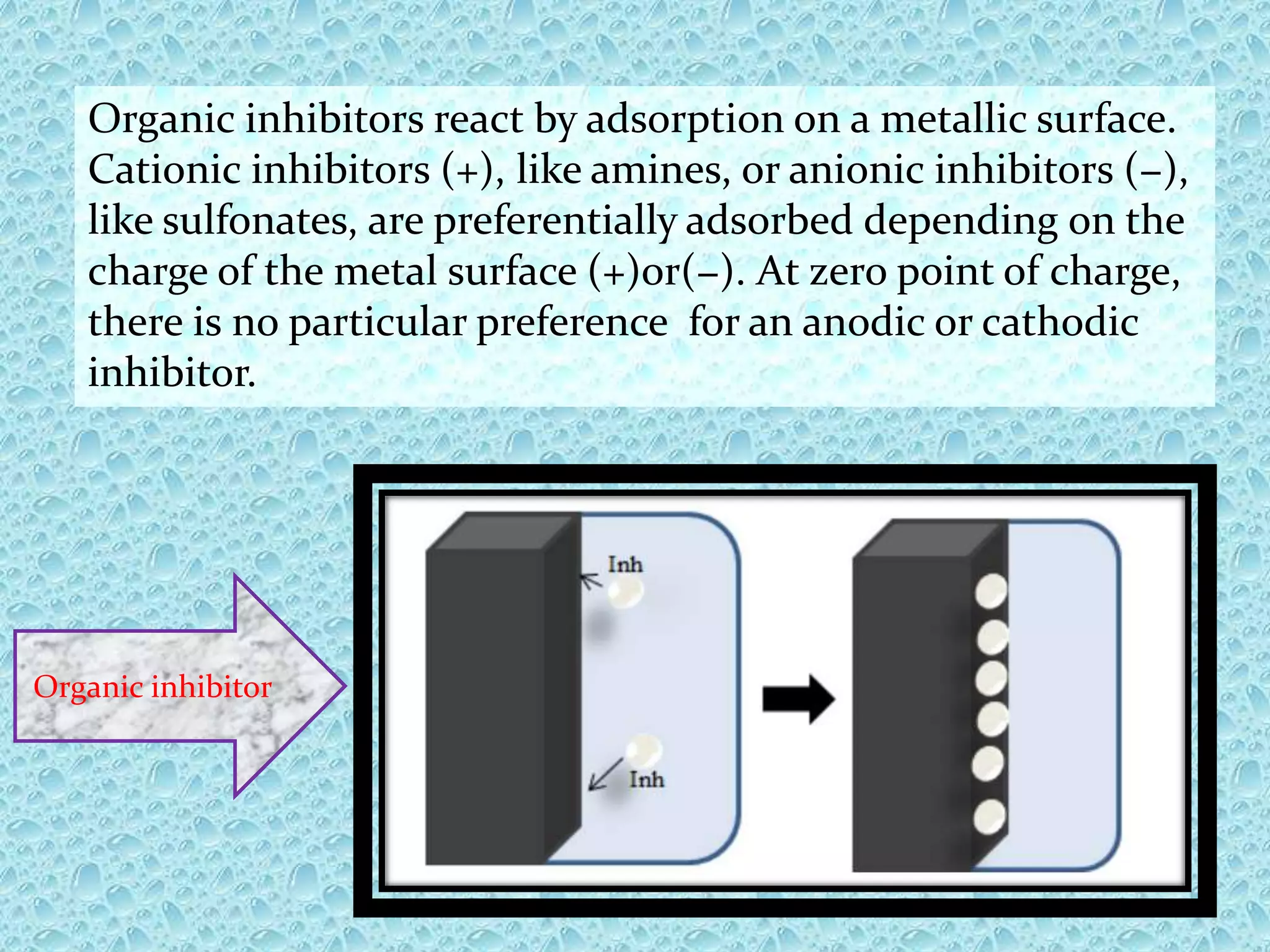
Corrosion inhibitors are chemical substances that minimize or prevent corrosion when added in small concentrations to an environment. They work by forming protective films on metal surfaces or reacting with corrosive components. Inhibitors can be inorganic, like chromates and nitrites, or organic compounds. They are applied through continuous injection, batch treatment, or squeeze treatment. The efficiency of an inhibitor depends on its concentration and ability to form protective barrier films on metals. Scavengers like hydrazine and sodium sulfite are also used to remove oxygen which promotes corrosion. Inhibitors find applications in various industries like petroleum, packaging, sour gas, and cooling systems.