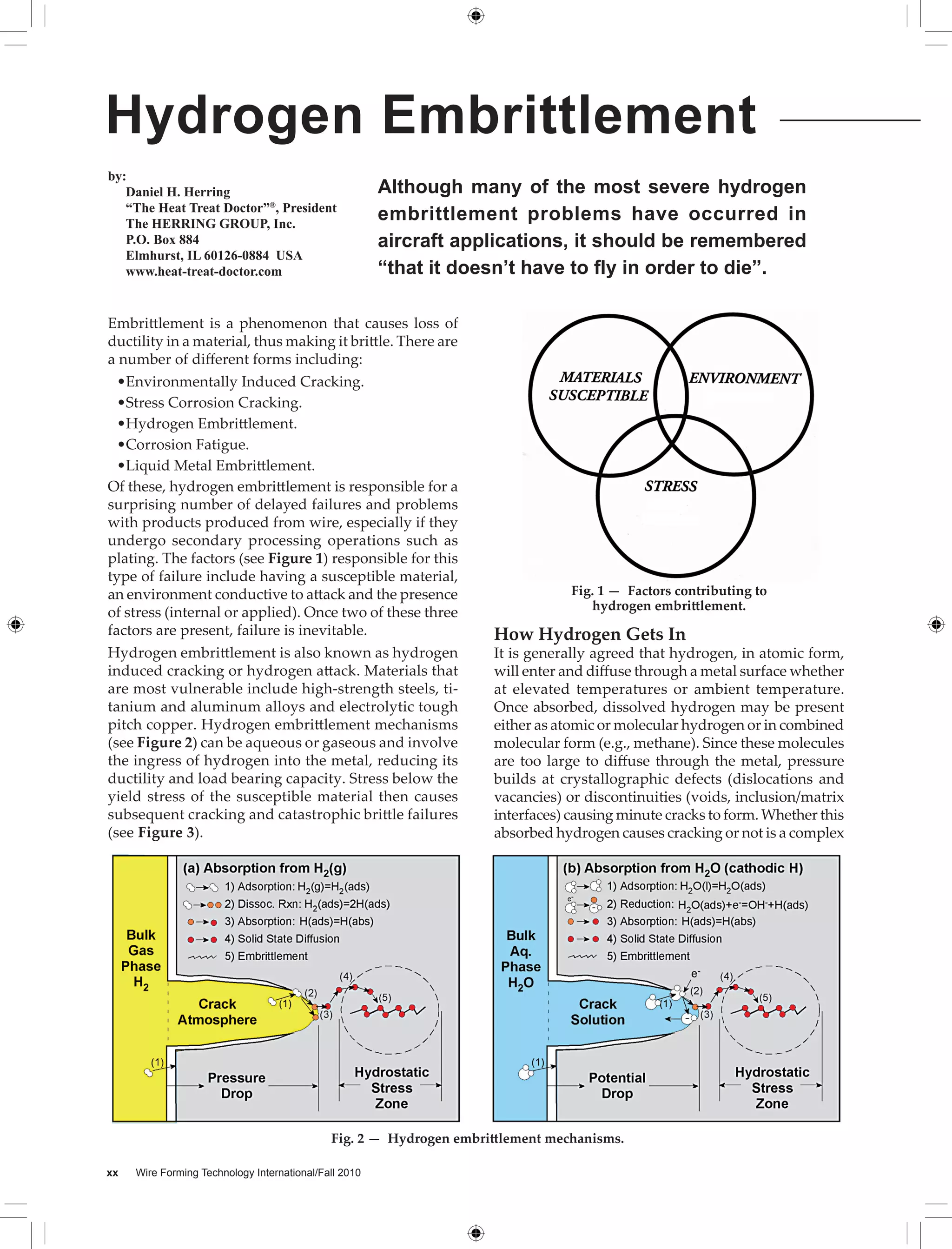
Hydrogen embrittlement is a phenomenon that causes metals like steel, titanium, and aluminum alloys to become brittle. It occurs when hydrogen enters these metals, reducing their ductility and load bearing capacity. There are several ways hydrogen can get into metals, such as from acid cleaning, electroplating, welding, and heat treating. Once hydrogen is absorbed, even small amounts below detection levels can cause cracking or failure of parts, especially under stress. Proper baking and controlling hydrogen exposure during manufacturing processes can help prevent failures from hydrogen embrittlement.