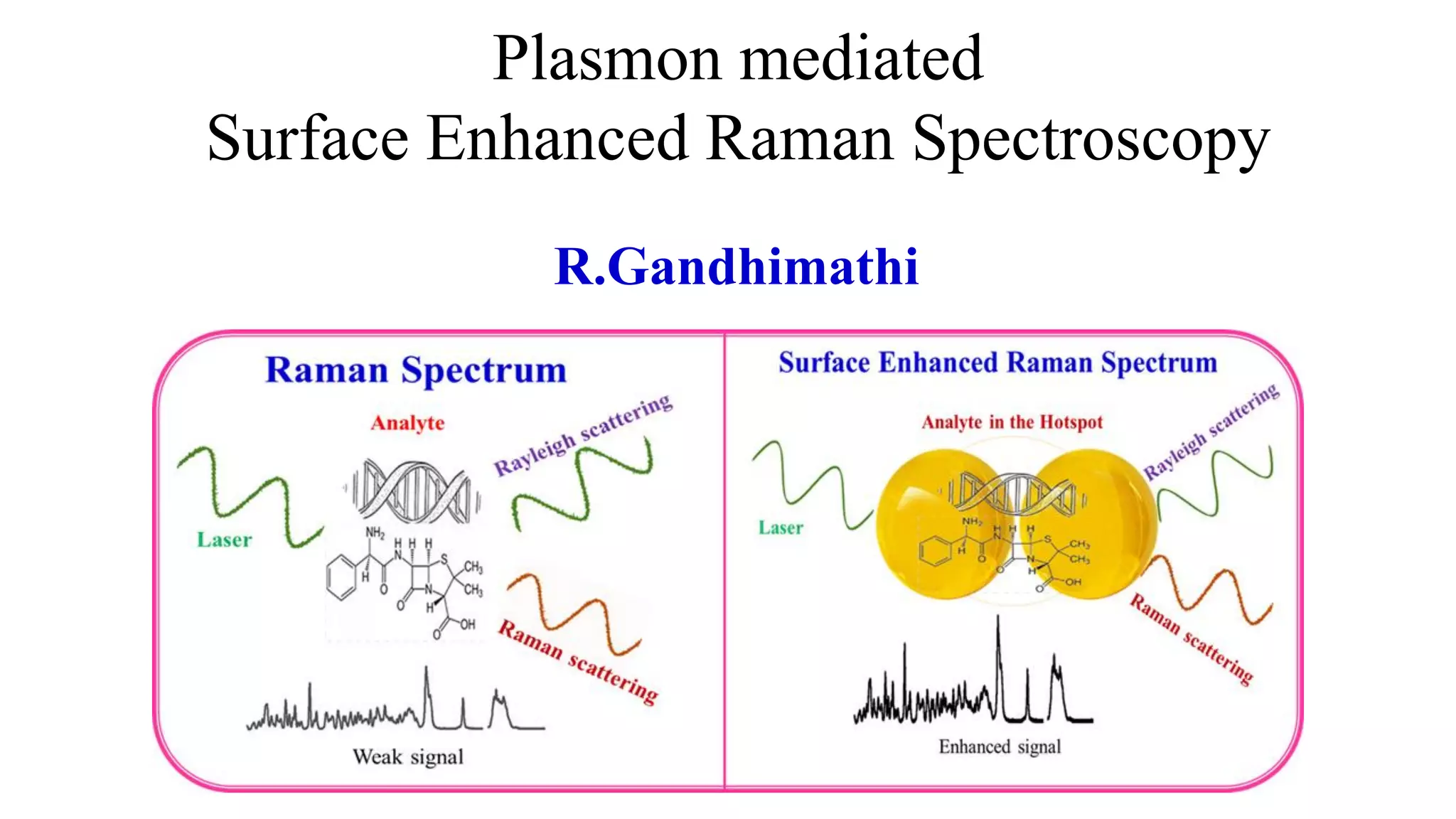
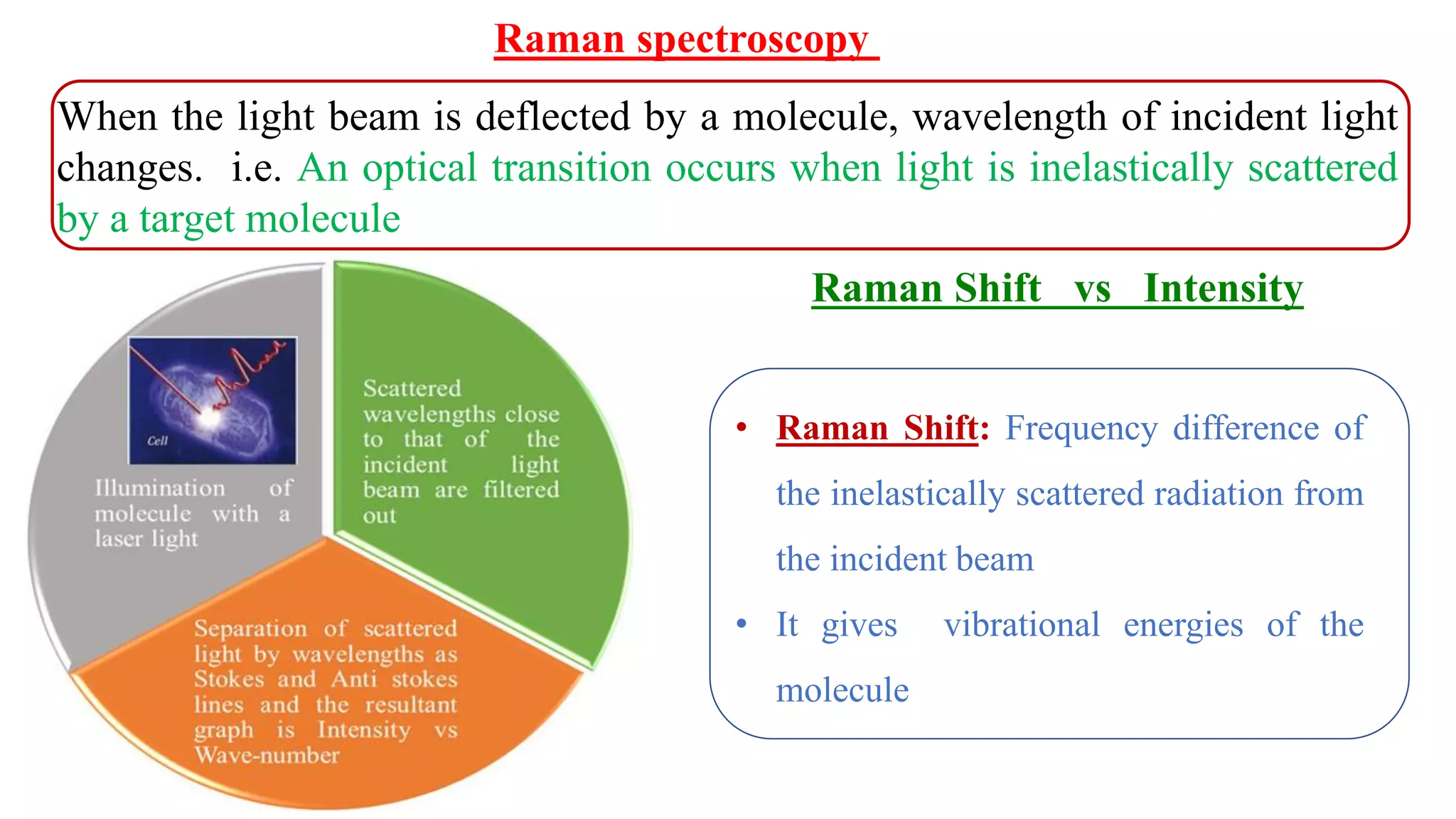
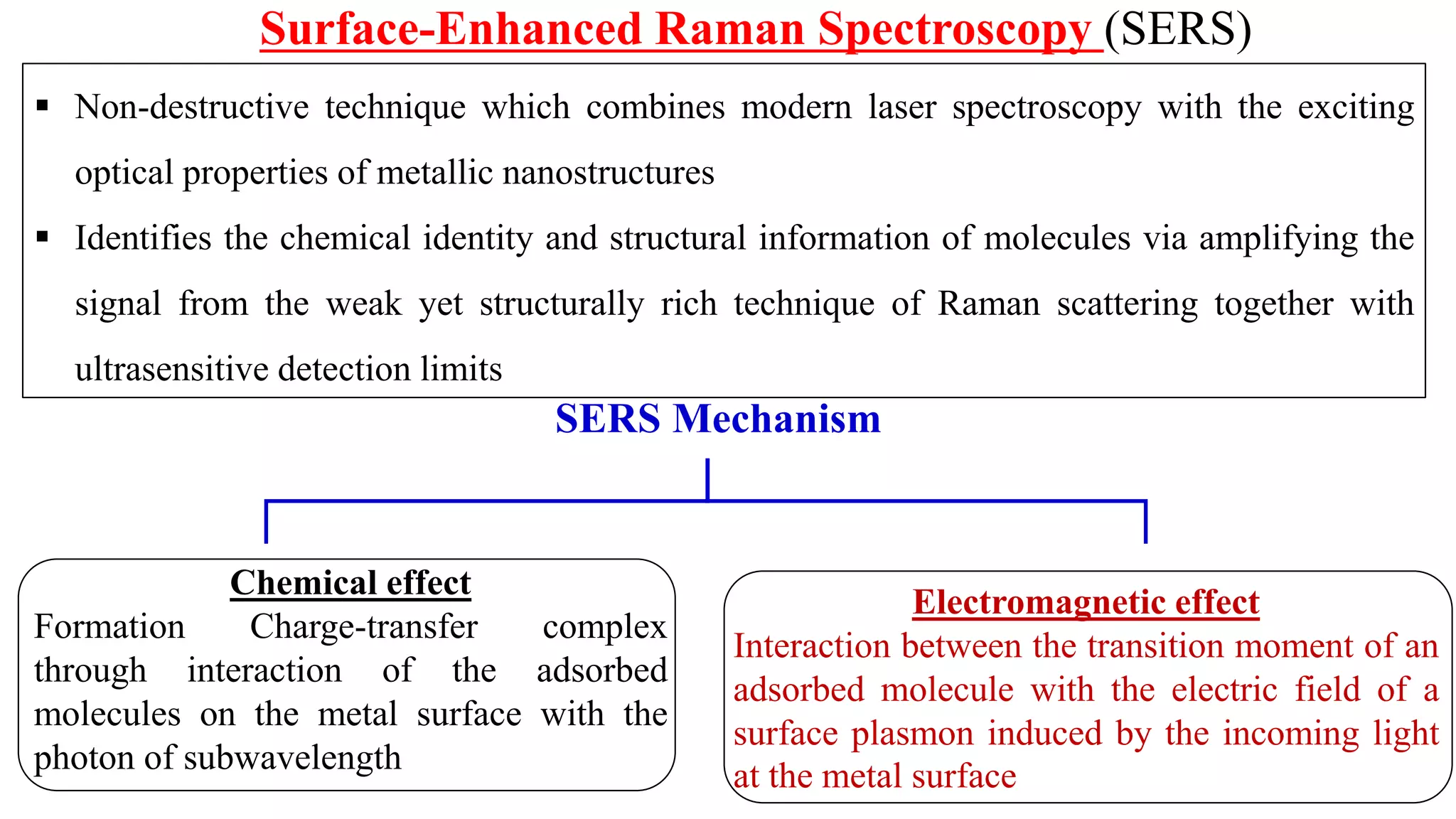
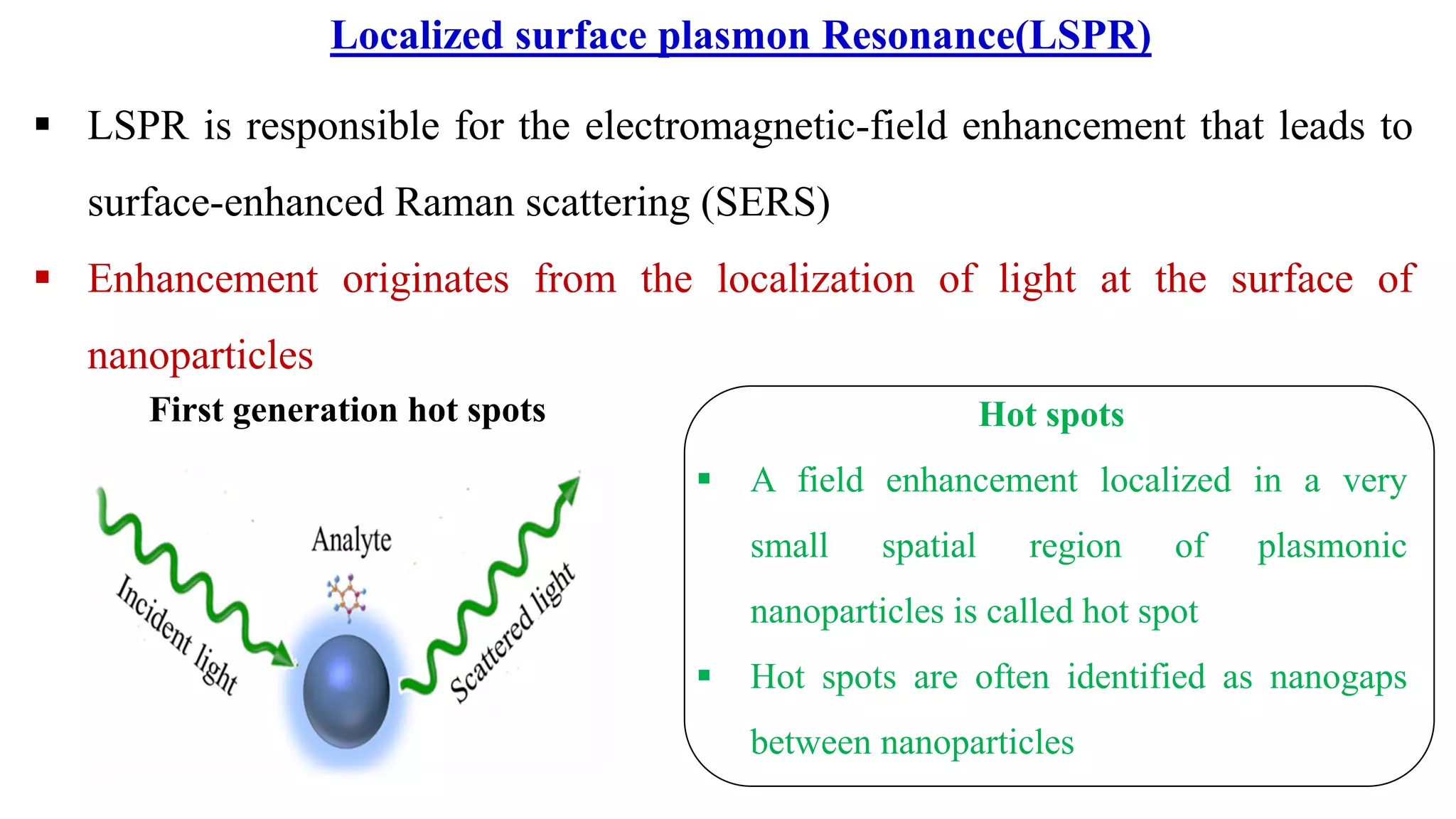
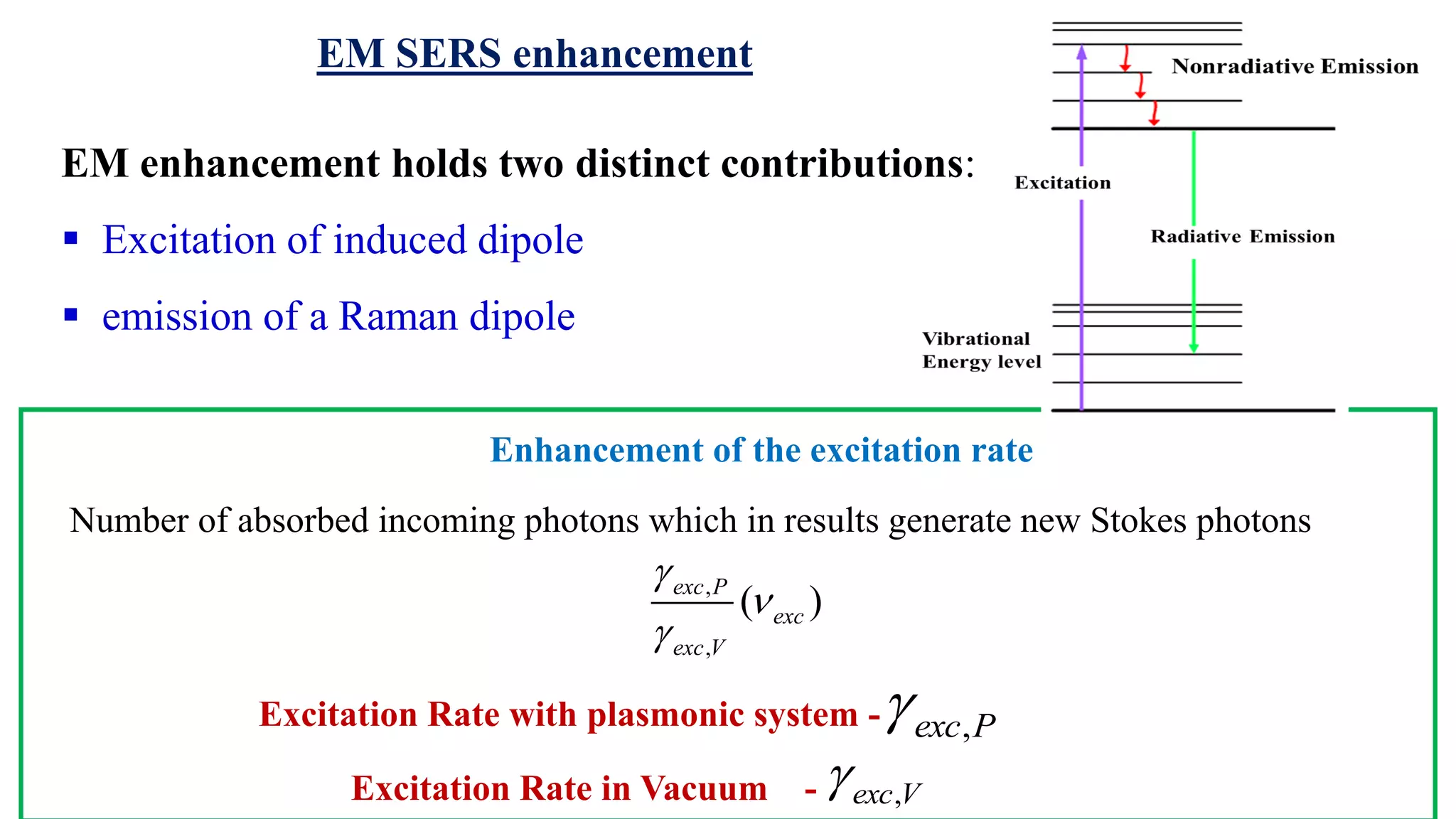
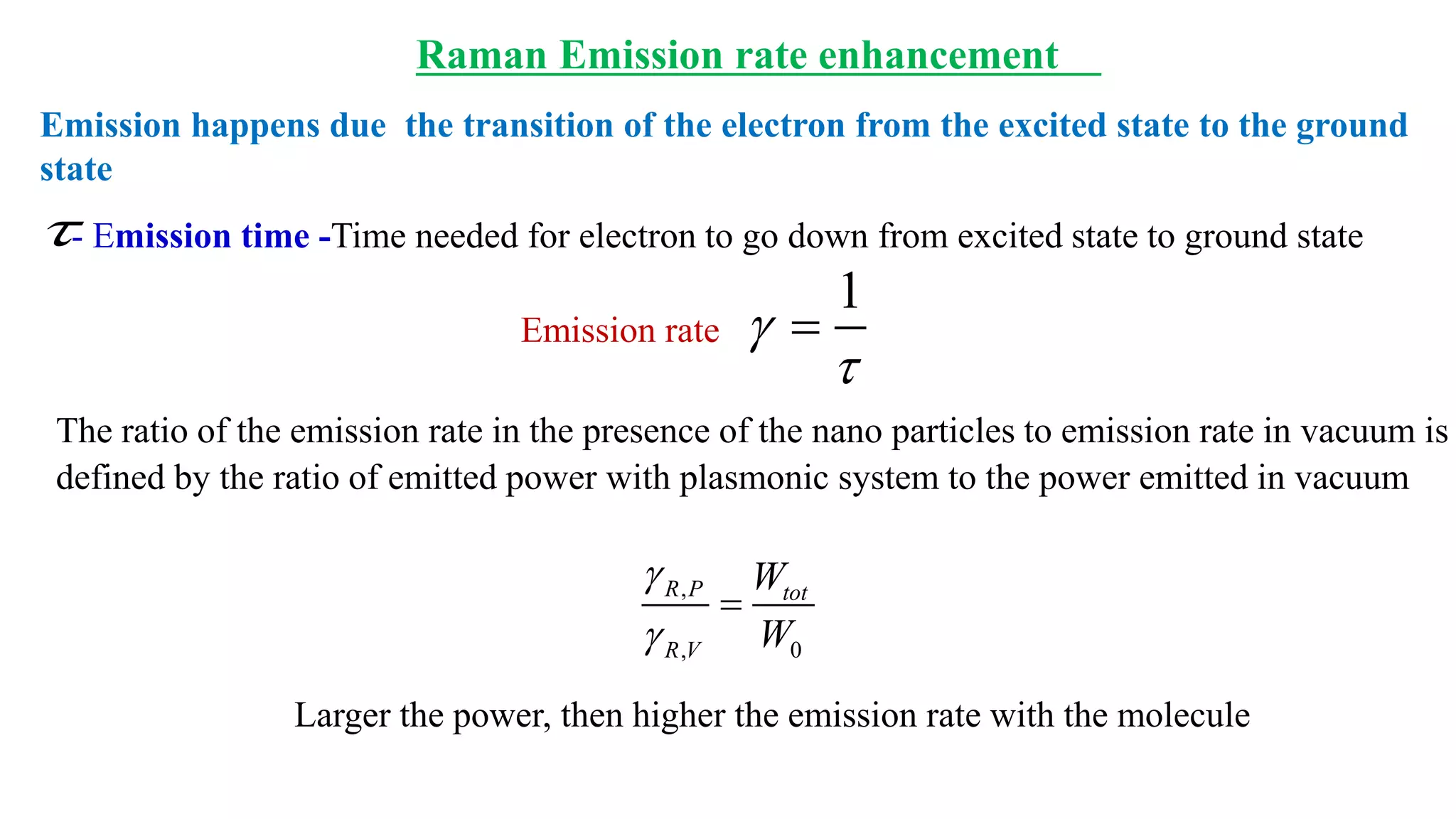
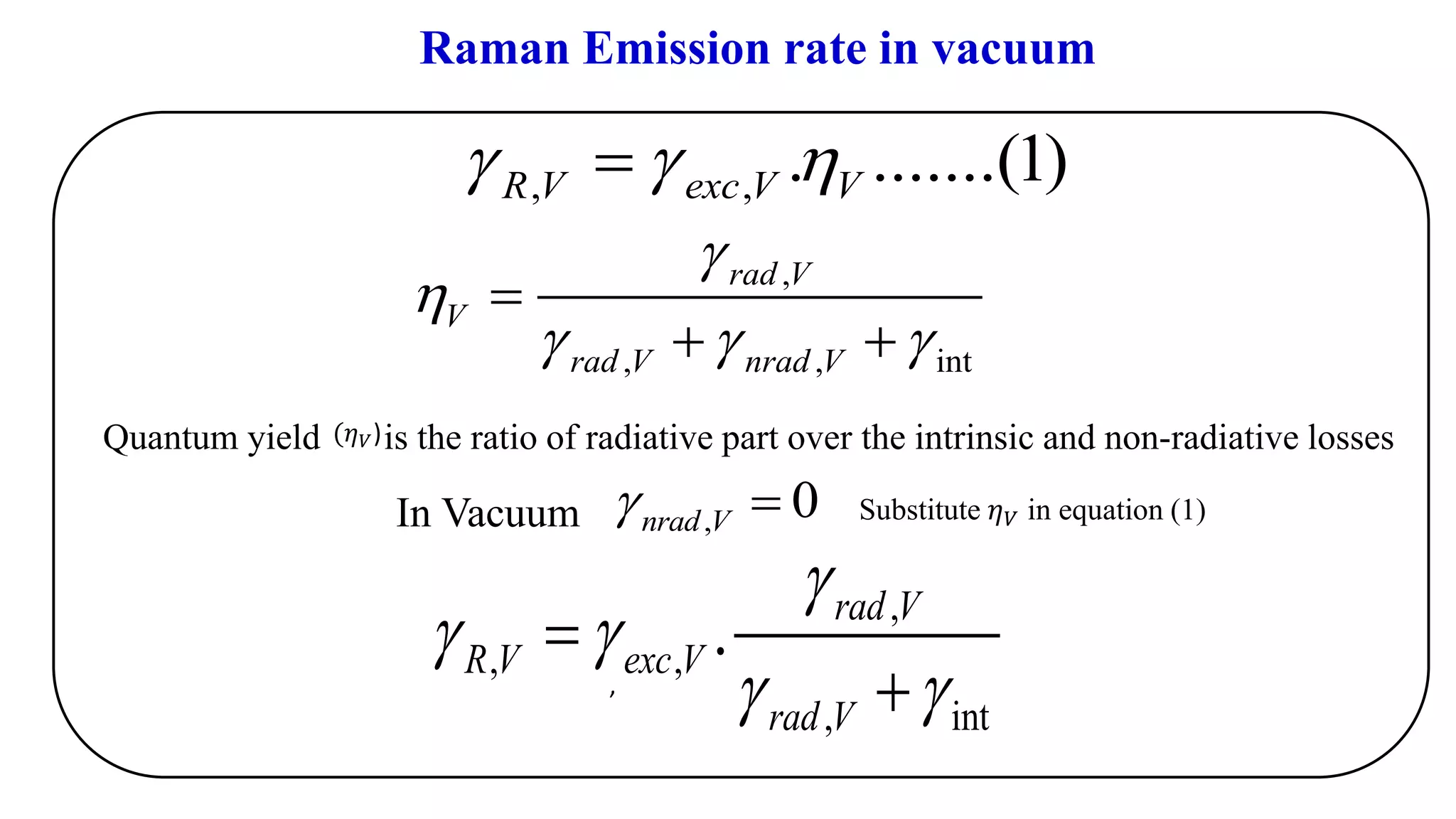
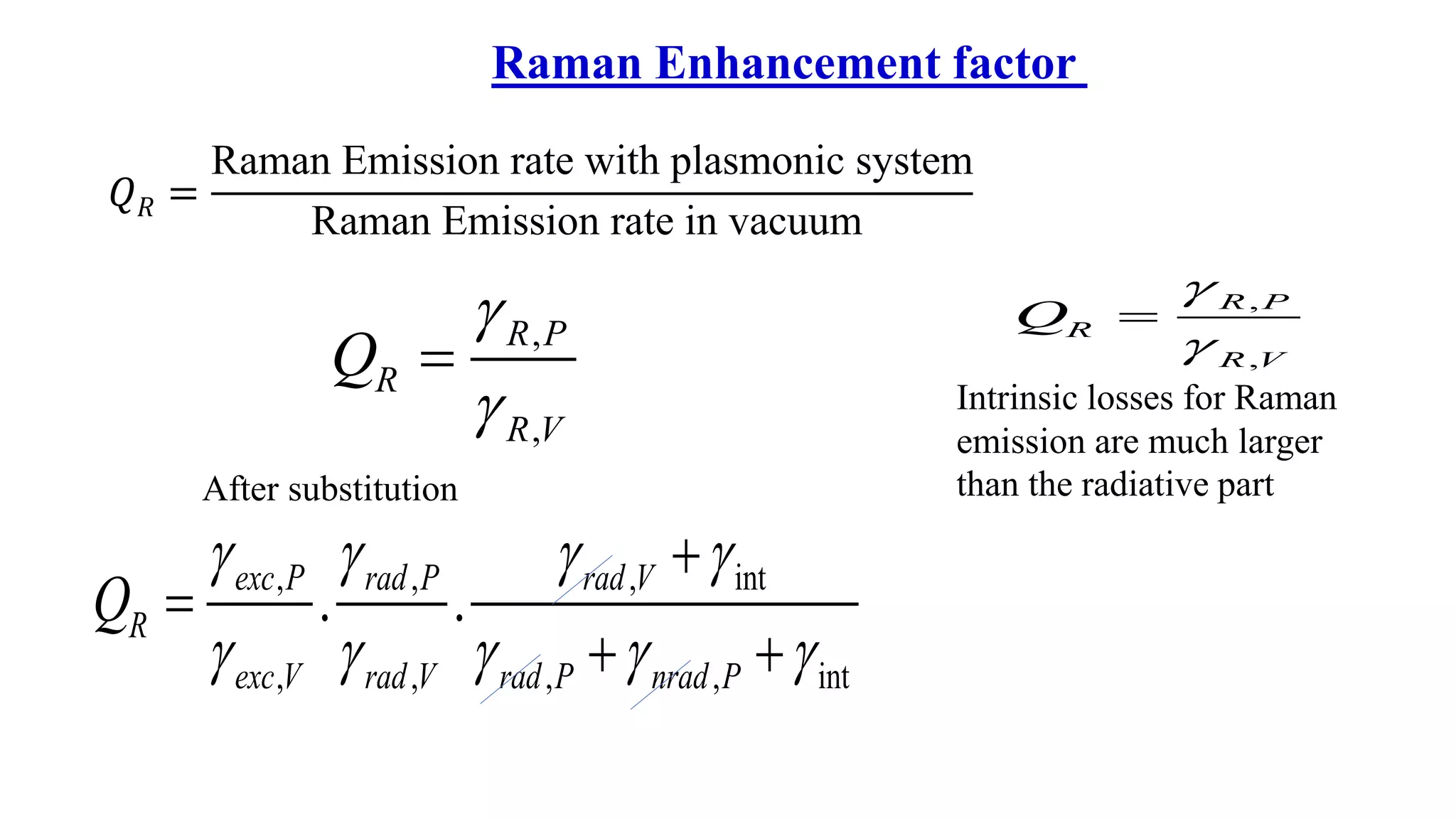
This document discusses surface enhanced Raman spectroscopy (SERS) and the mechanisms that lead to signal enhancement. It explains that SERS combines Raman spectroscopy with localized surface plasmon resonance on metallic nanostructures to amplify the weak Raman signal from molecules up to 1011 times. This electromagnetic enhancement is due to the localized electric fields that excite incident photons and enhance molecular emission. Hotspots between nanoparticle gaps produce particularly large field enhancements. The document outlines excitation rate enhancement, emission rate enhancement, and overall SERS enhancement factor calculations.