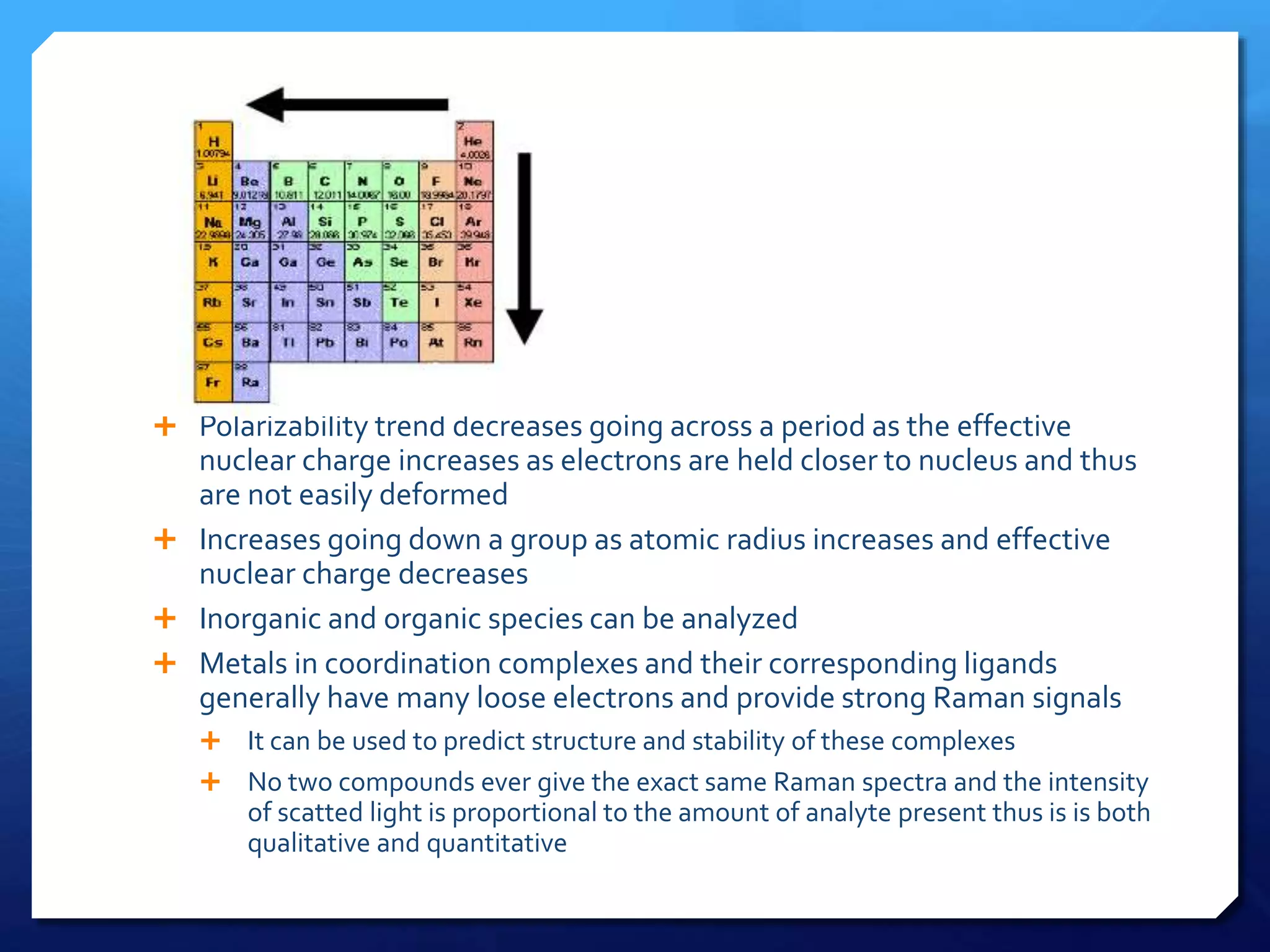
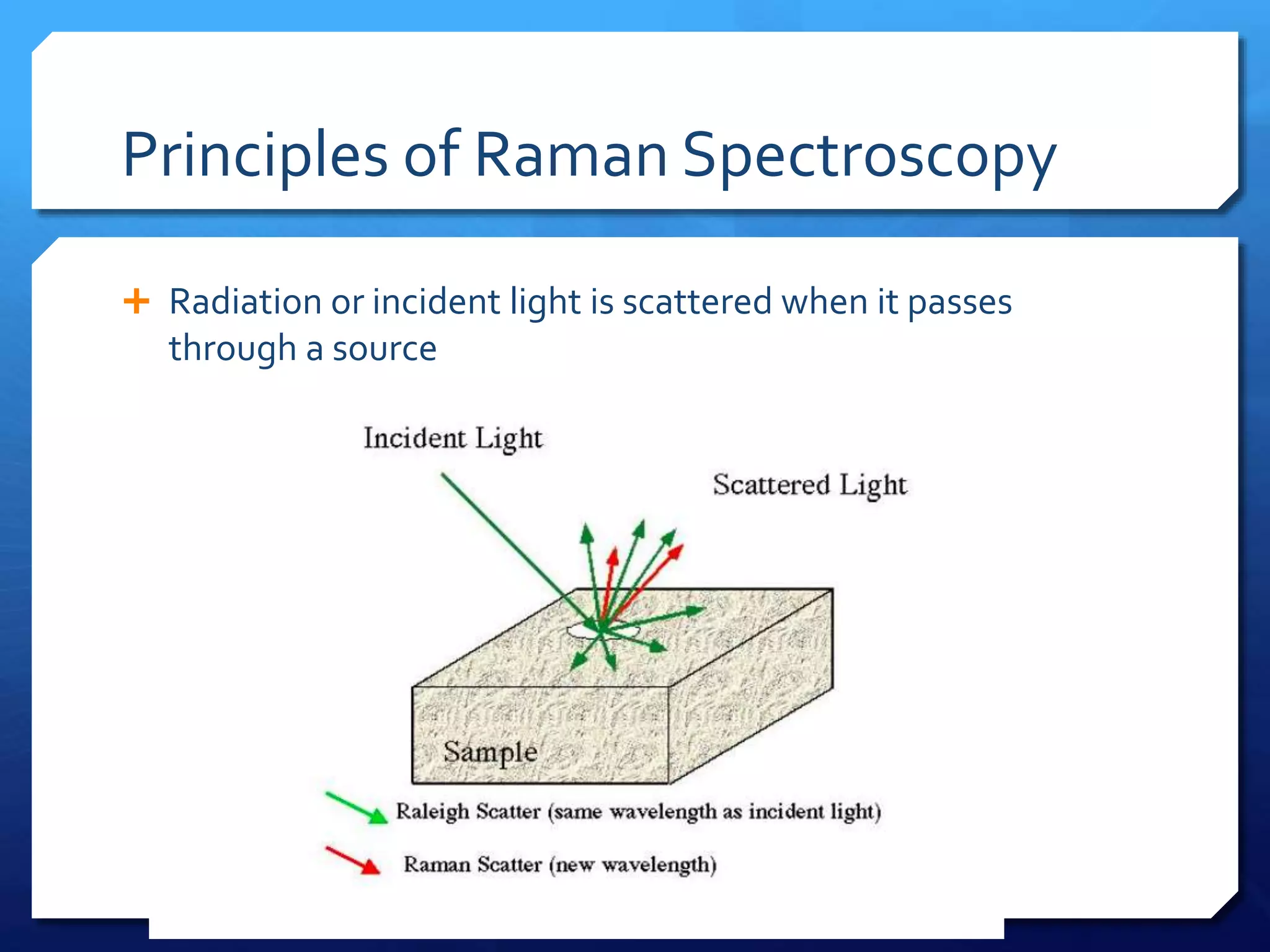
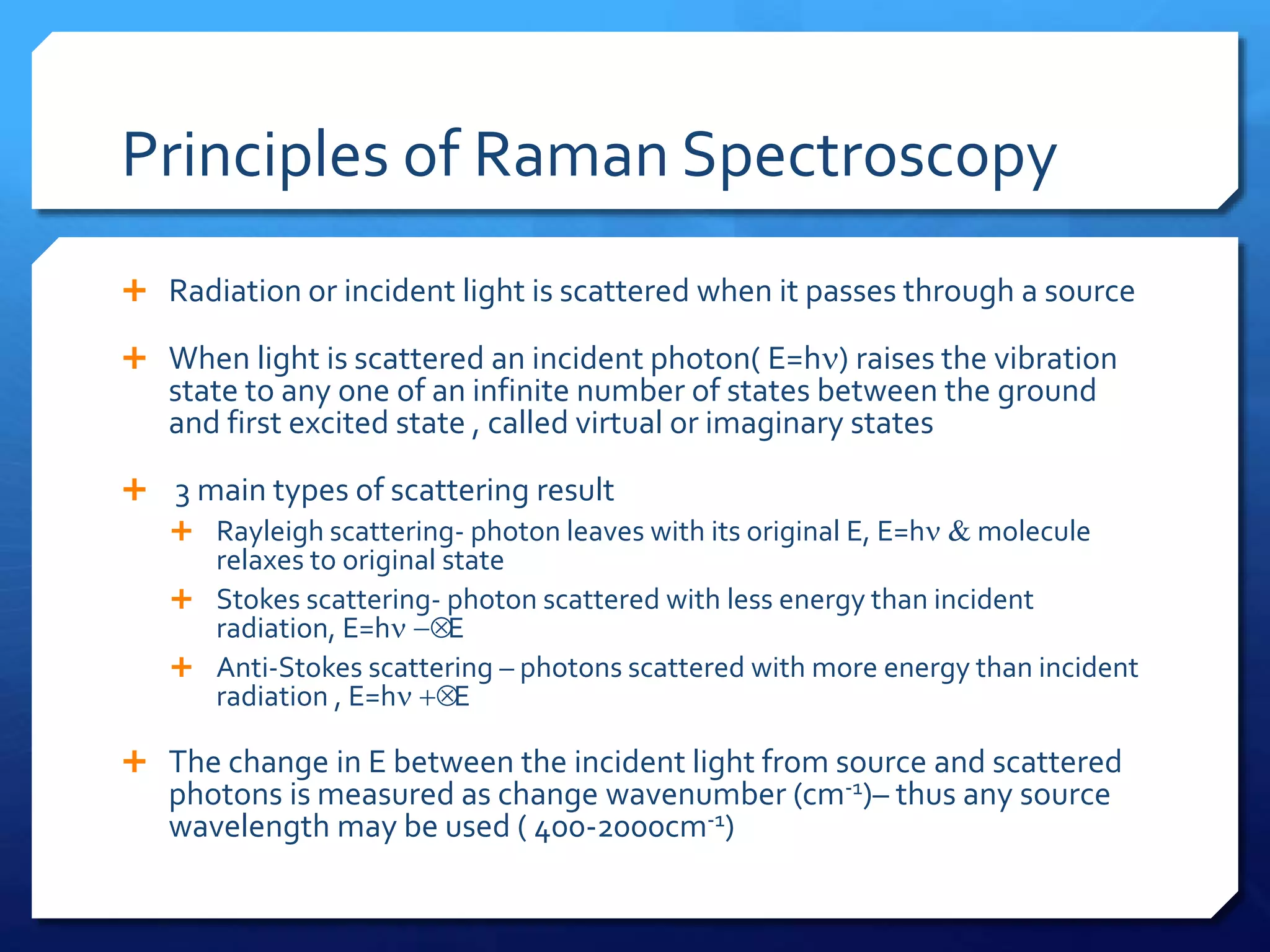
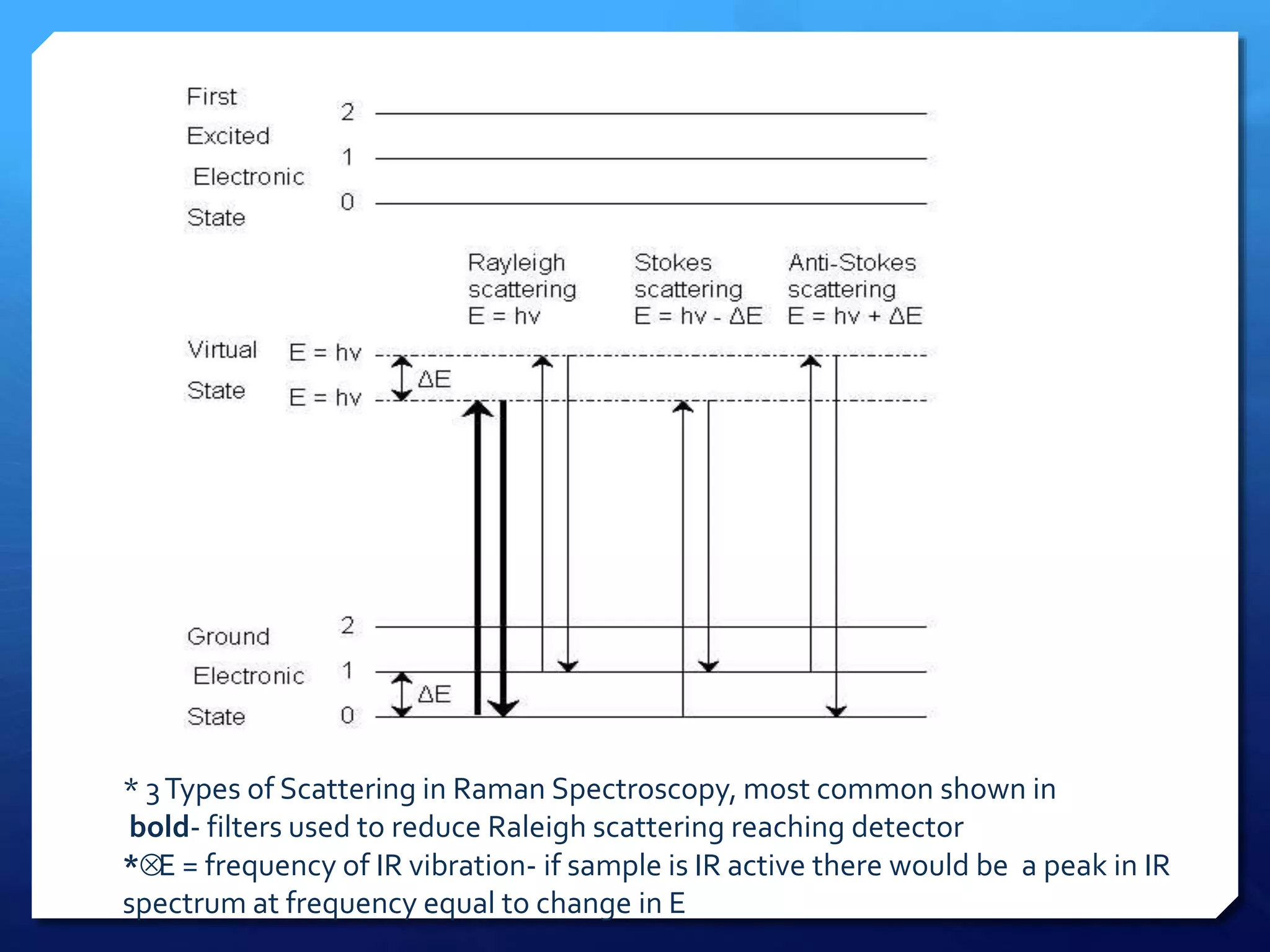
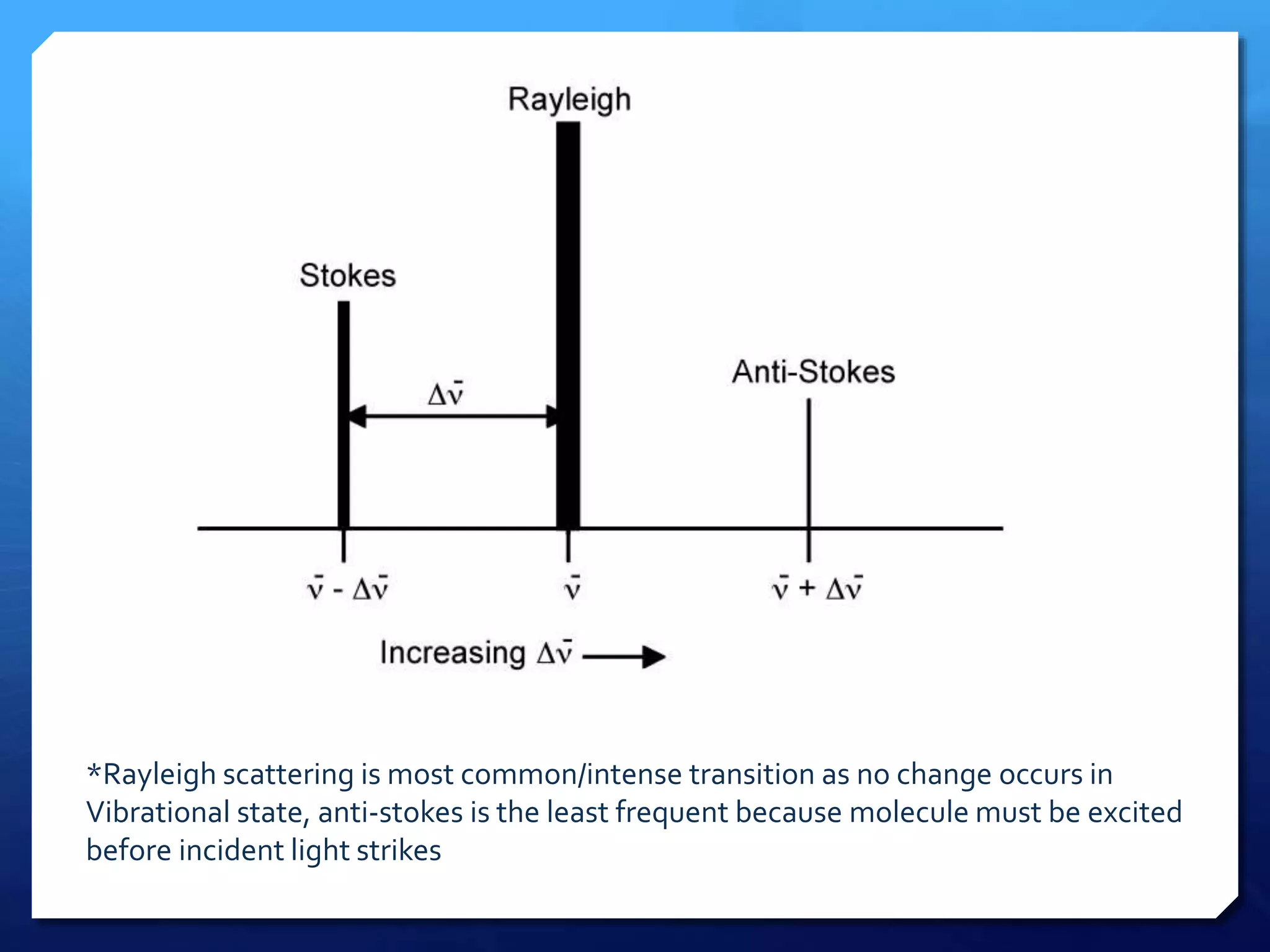
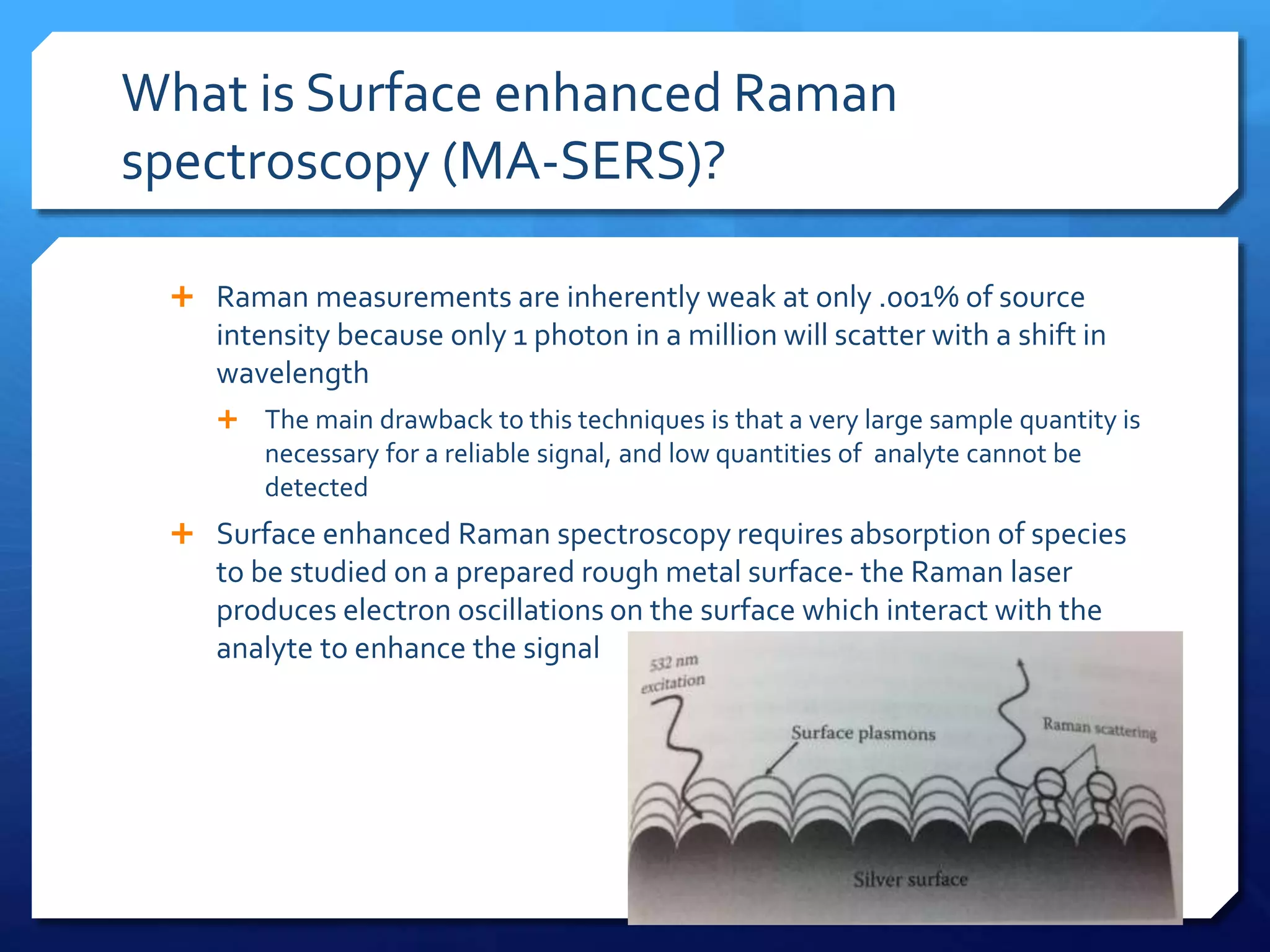
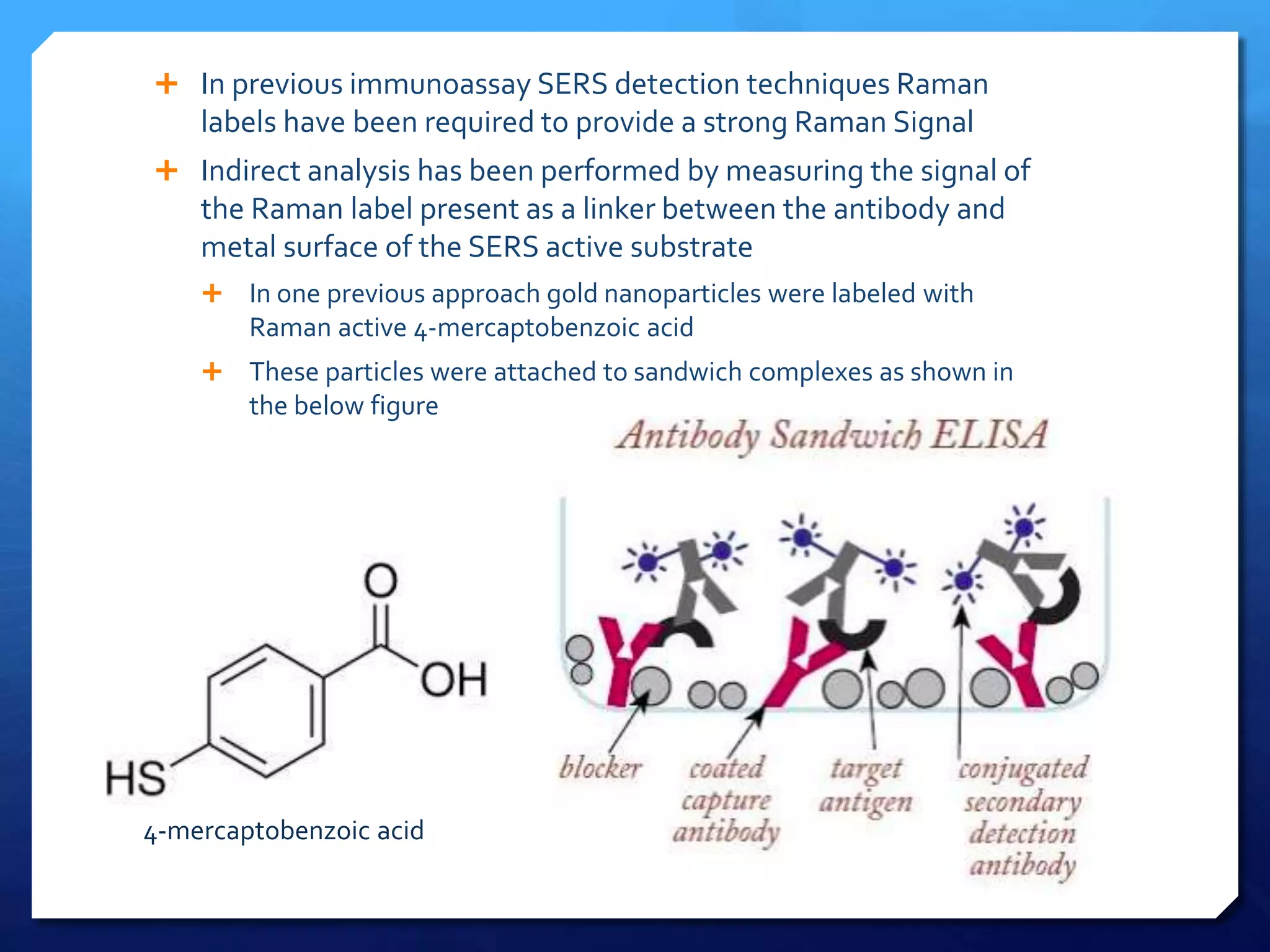
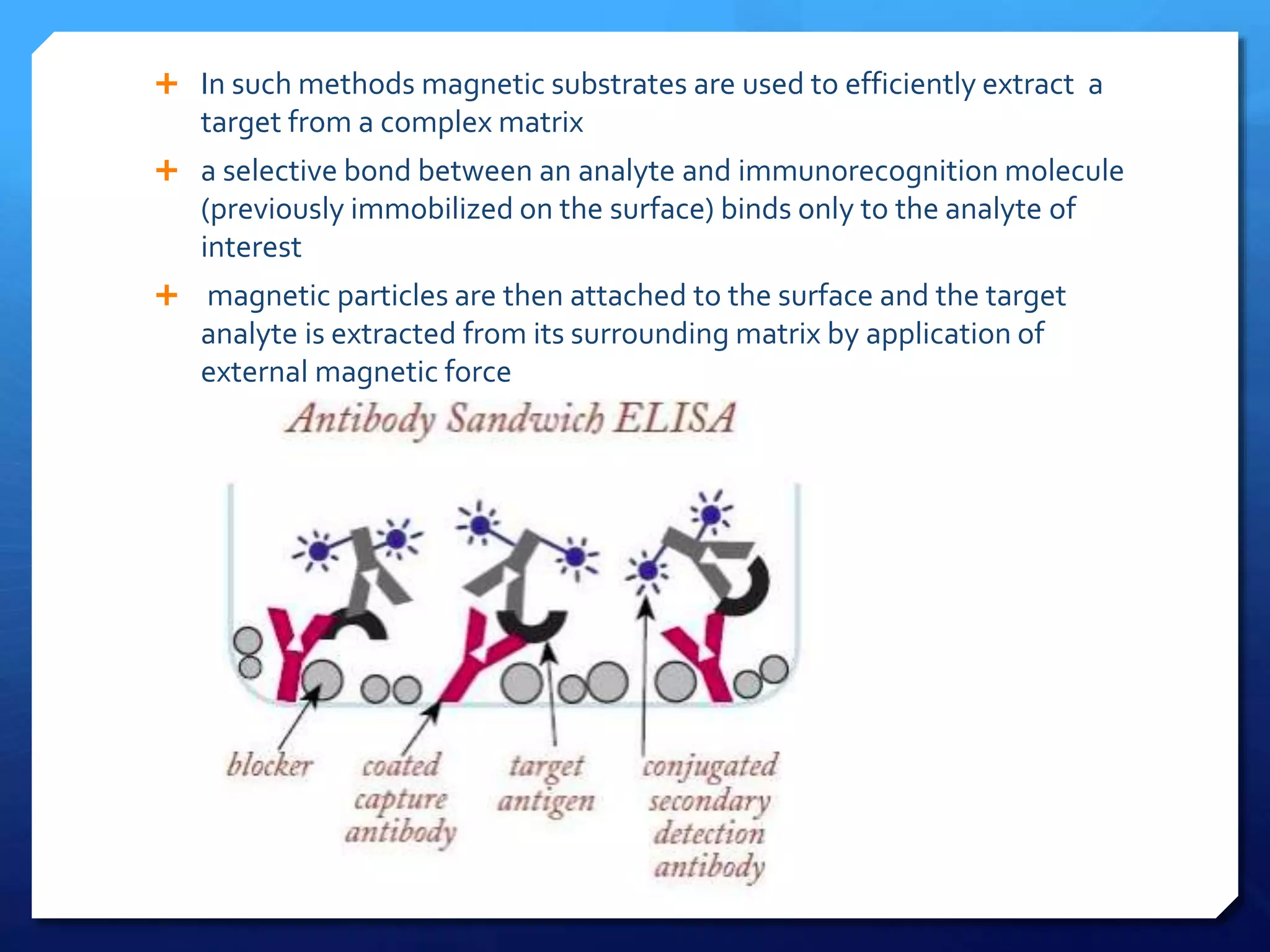
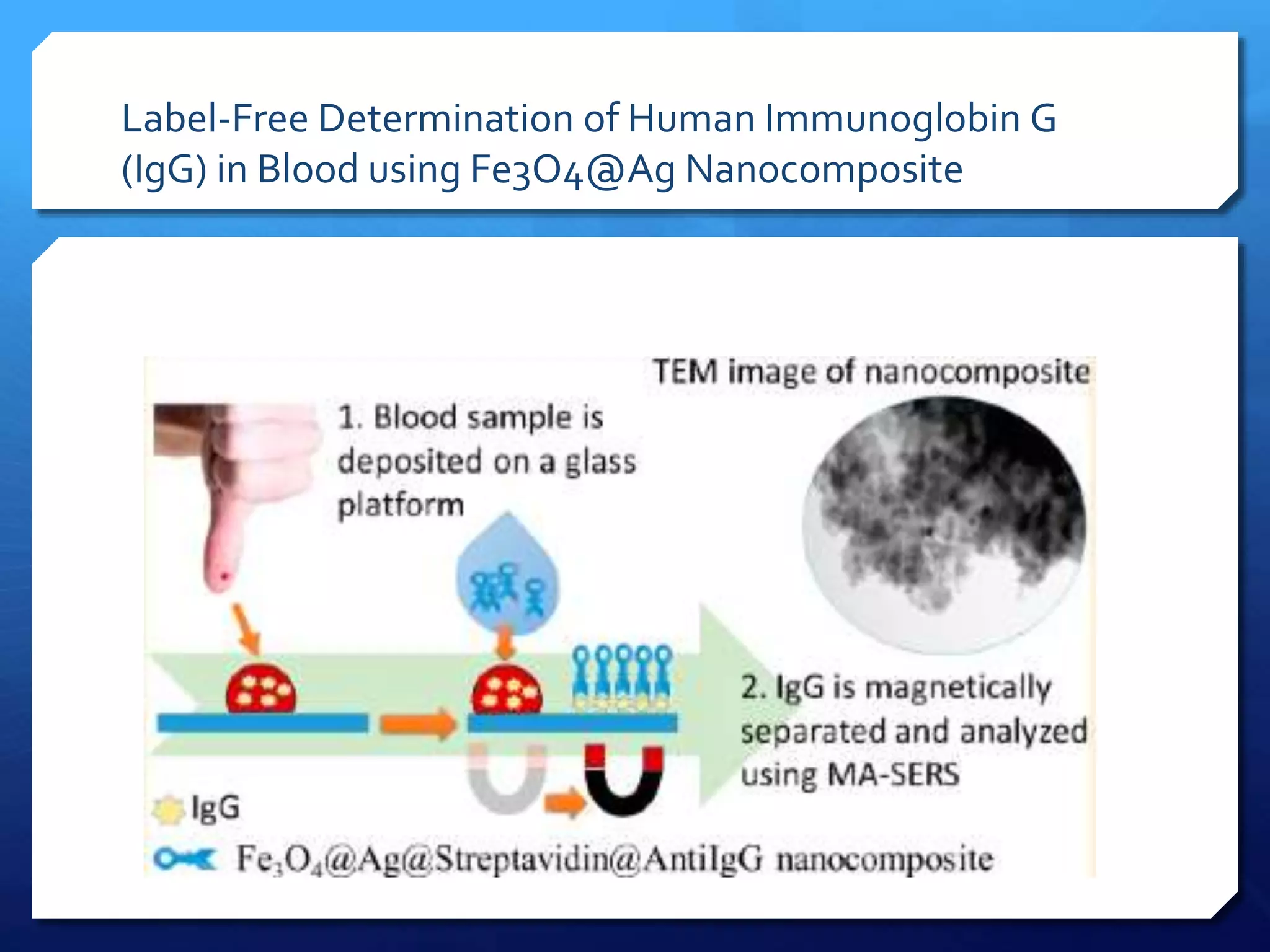
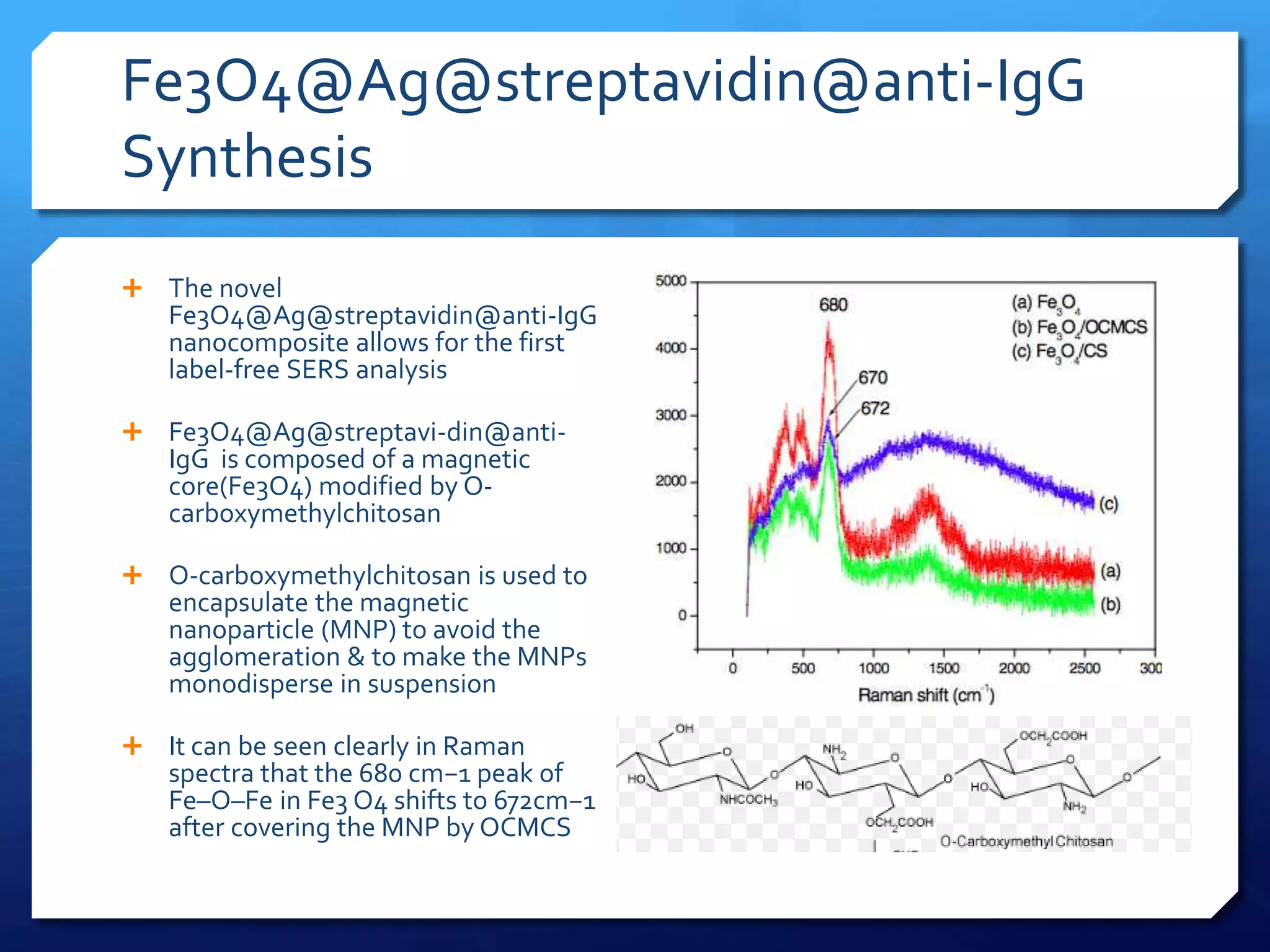
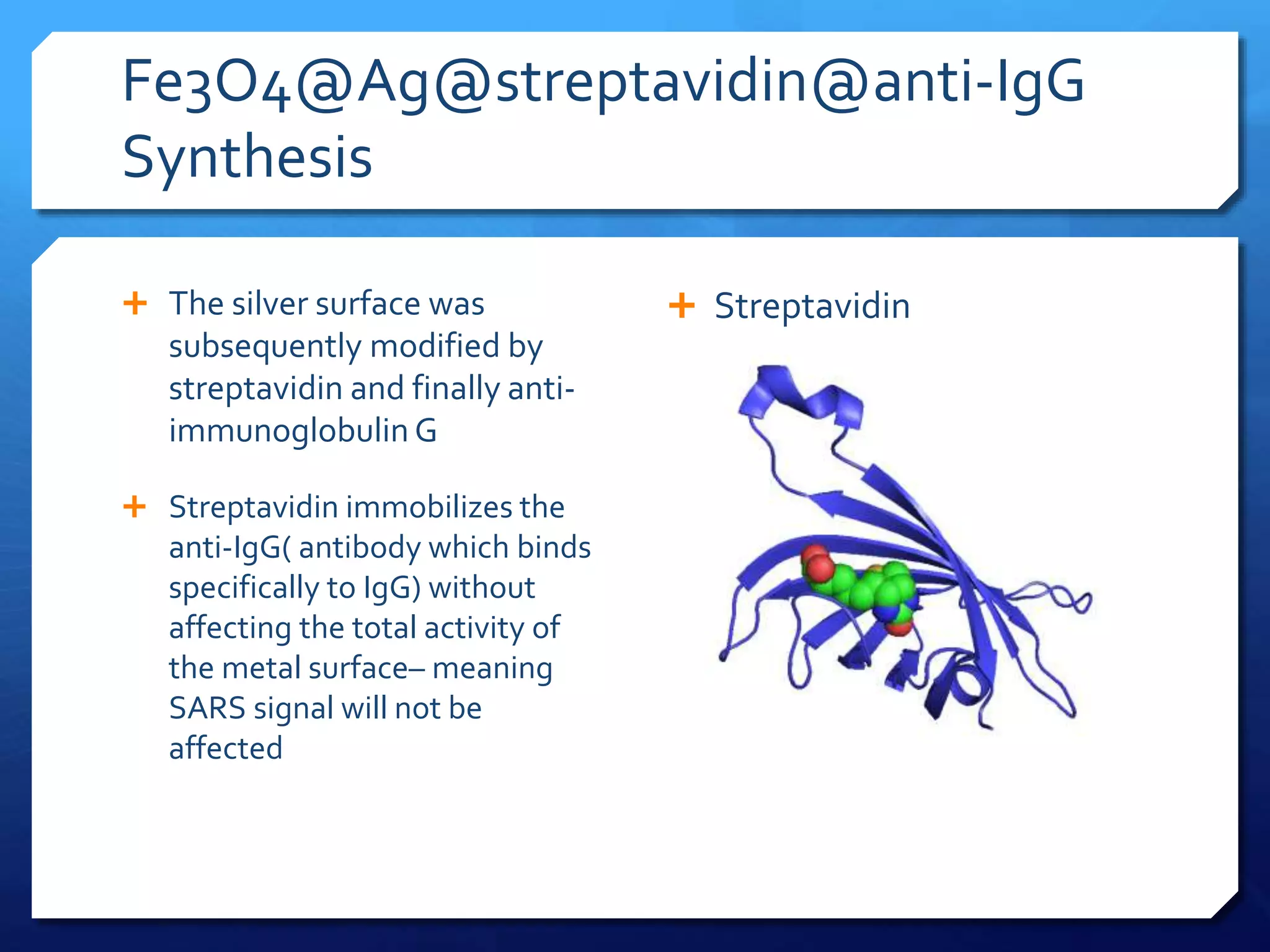
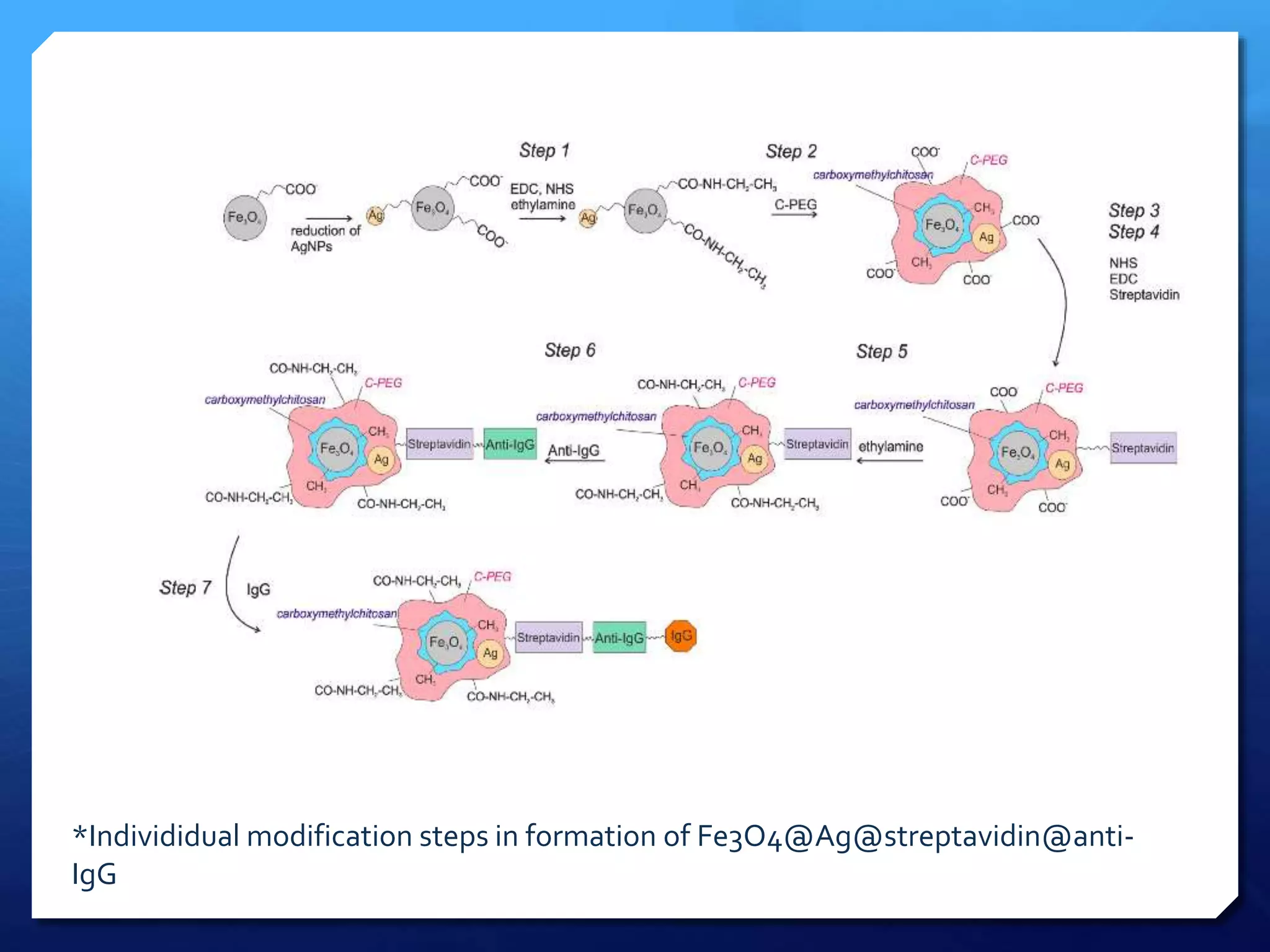
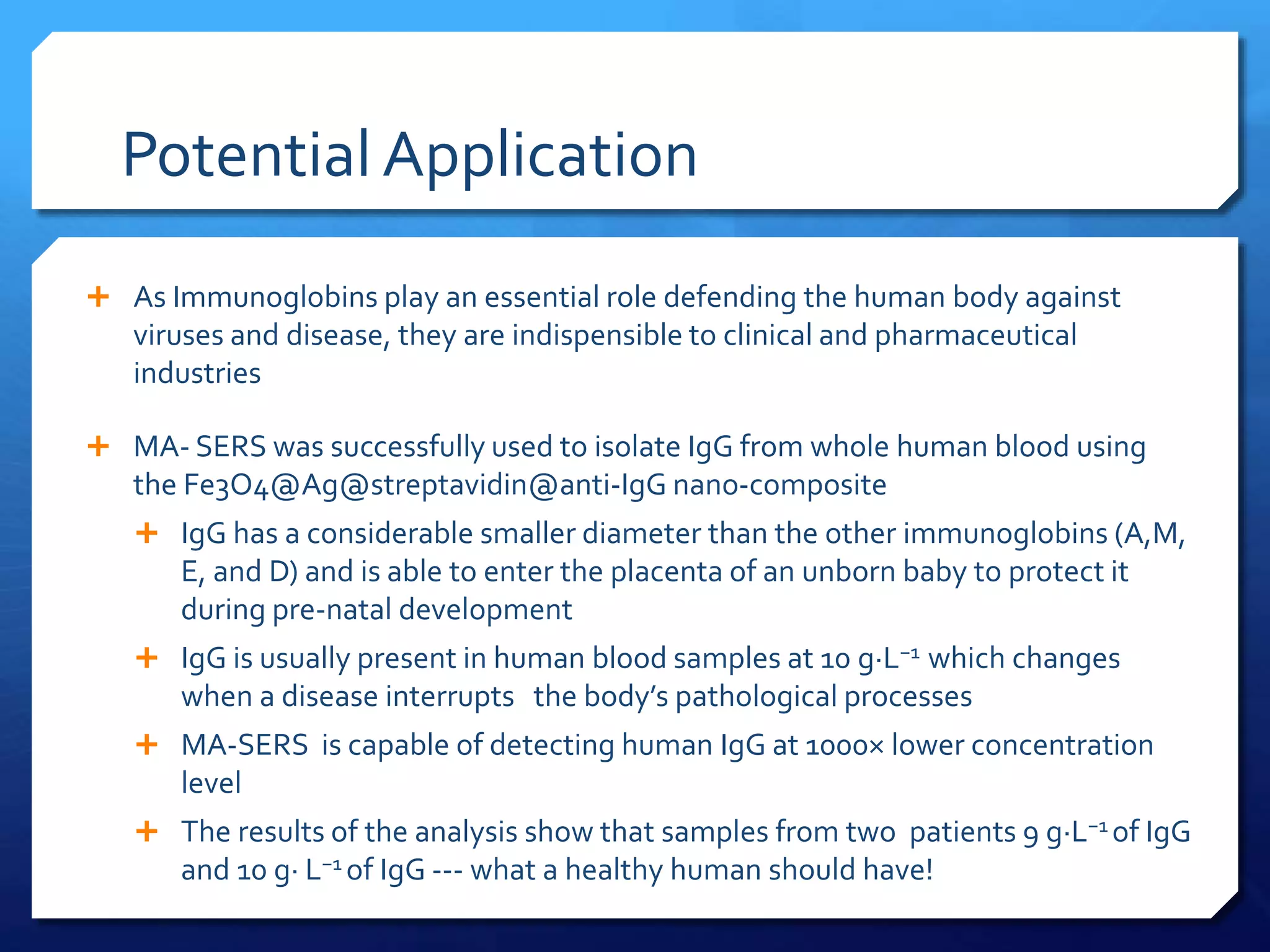
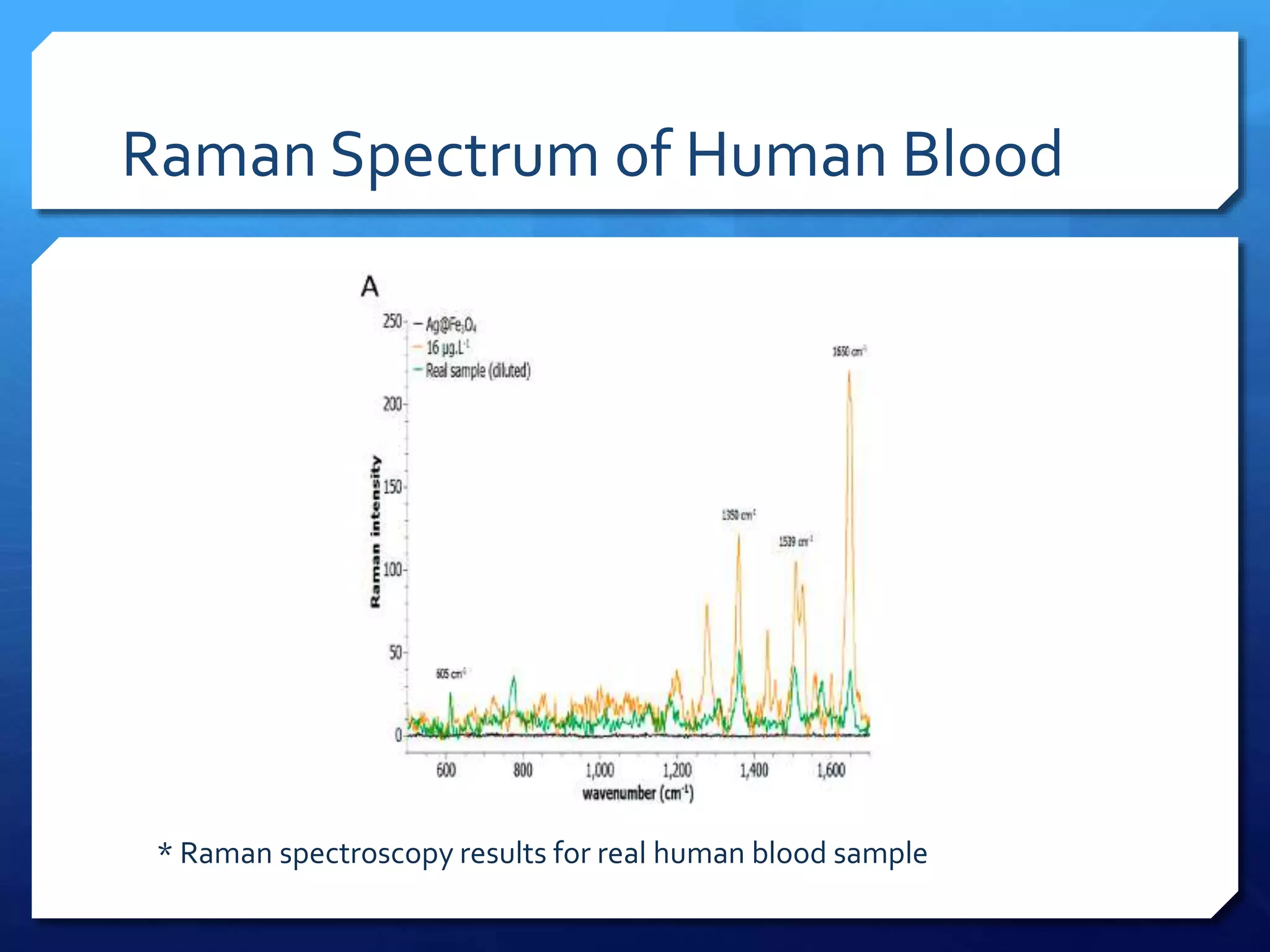
The document discusses magnetically assisted surface enhanced Raman spectroscopy (MA-SERS) and its potential clinical applications. It describes how MA-SERS uses a magnetic nanocomposite containing iron oxide and silver nanoparticles to isolate analytes from complex matrices and enable highly sensitive label-free detection. As an example, MA-SERS was used to detect human immunoglobin G at concentrations 1000 times lower than standard levels in whole blood samples. The document concludes that developing MA-SERS capabilities could help companies like Thermo Scientific better compete in the Raman spectroscopy market and support important medical research.