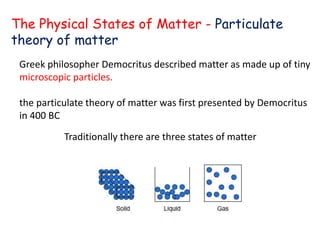
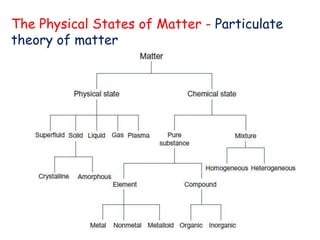
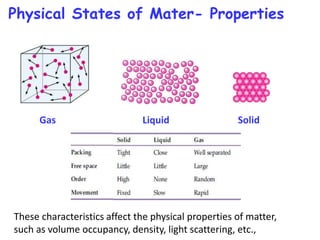
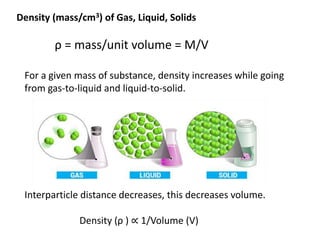
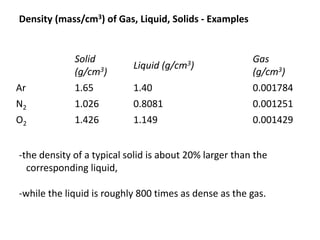
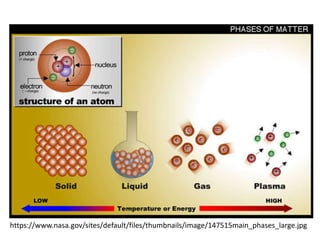
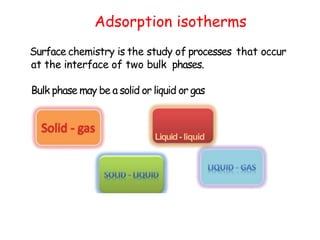
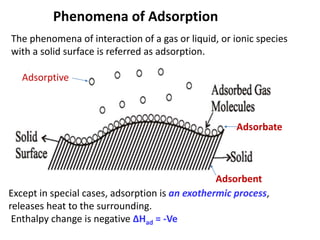
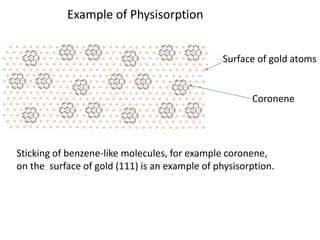
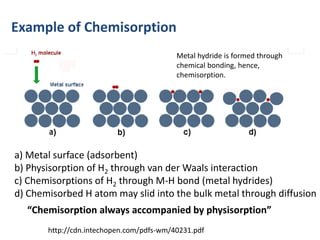
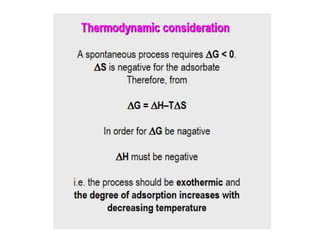
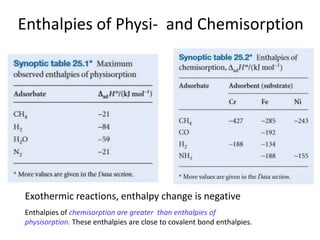
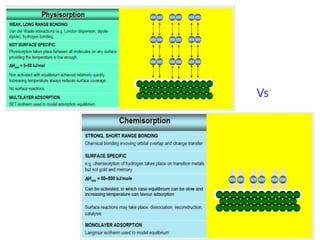
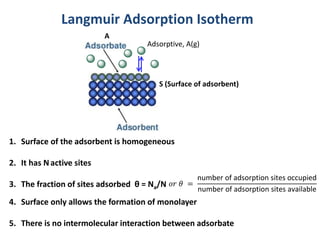
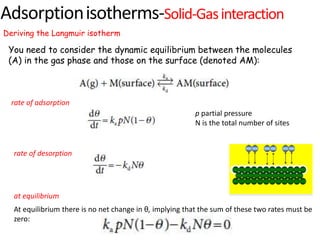
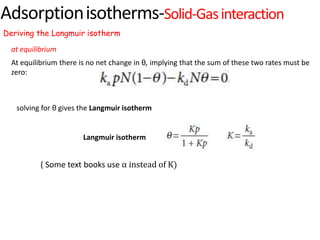
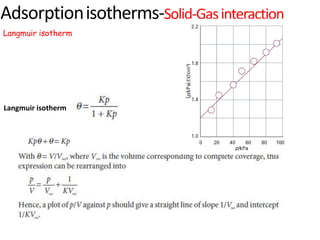
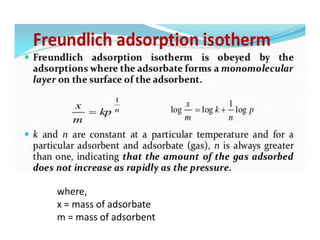
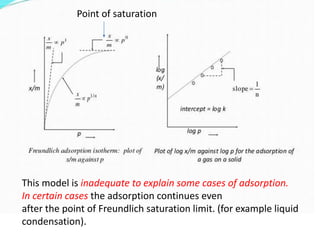
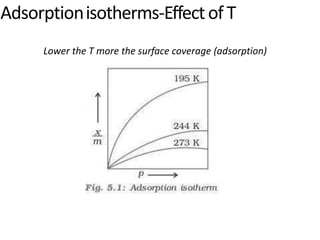
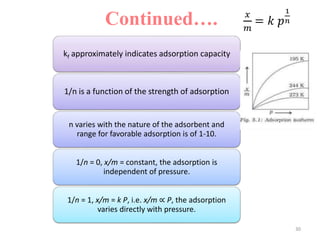
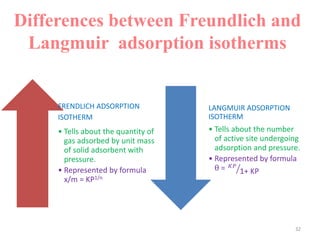
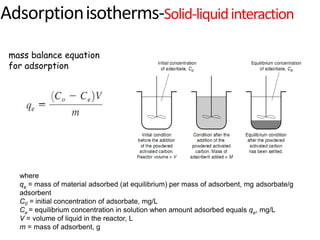
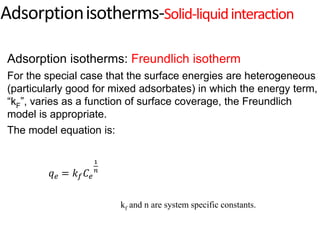
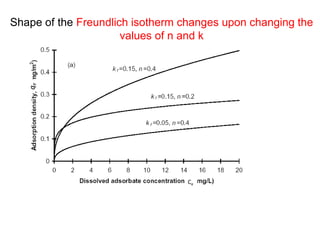
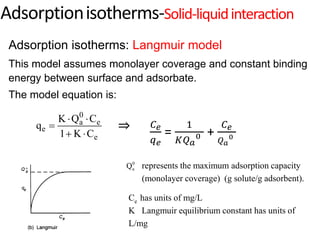
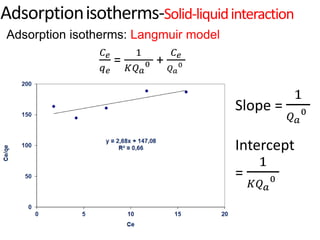
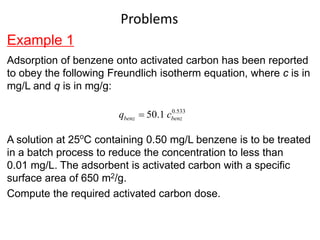
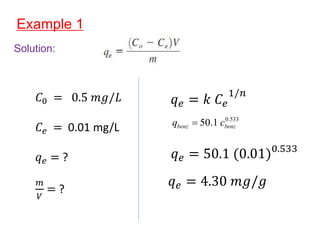
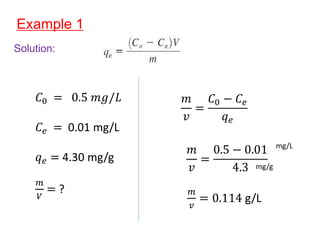
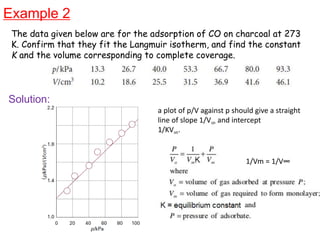
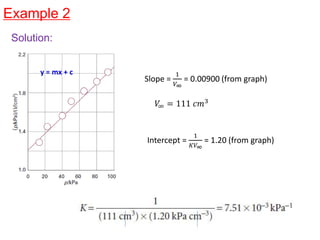
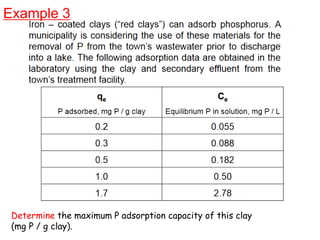
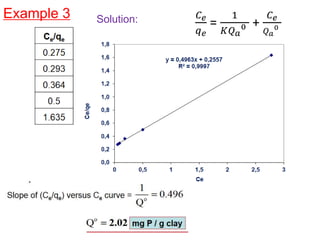
The document discusses various topics related to states of matter and adsorption isotherms. It begins by explaining the particulate theory of matter proposed by Democritus and developed by Dalton. It then describes the three traditional states of matter - solid, liquid, and gas - and how their properties, such as density, differ. A fourth state of matter, plasma, is also introduced. The document then discusses adsorption isotherms and models such as Langmuir and Freundlich isotherms that describe the relationship between gas or liquid concentration and adsorption. Examples are provided to illustrate applying these isotherm models.