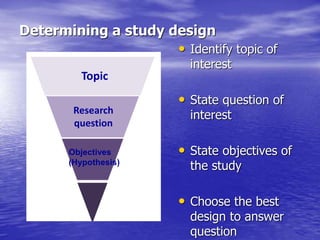
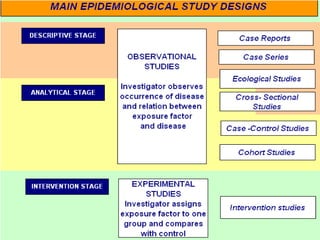
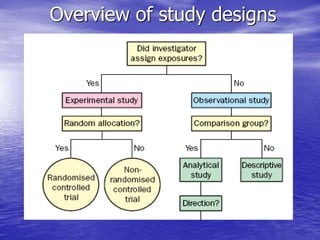
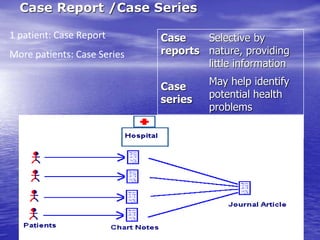
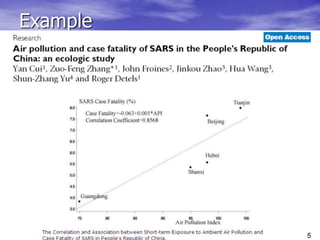
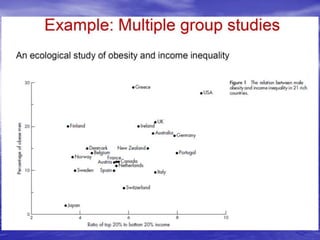
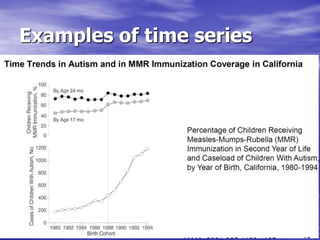
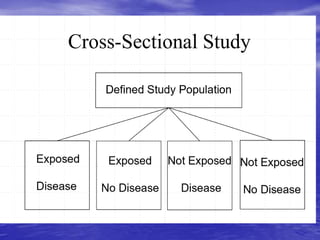
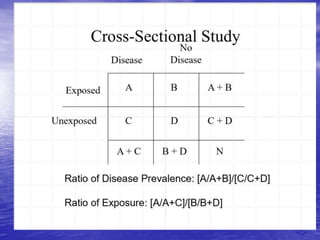
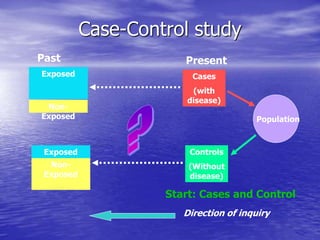
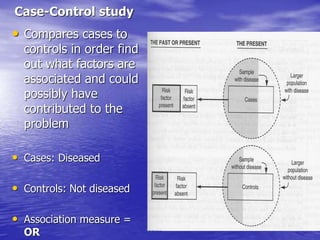
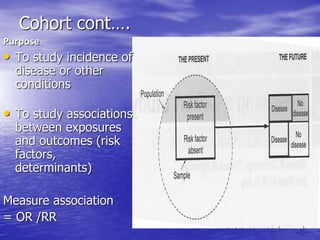
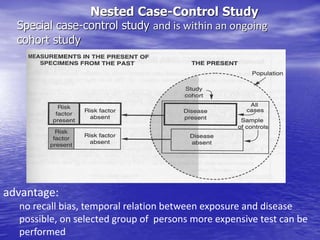
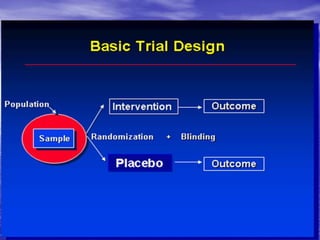
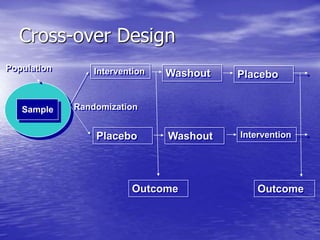
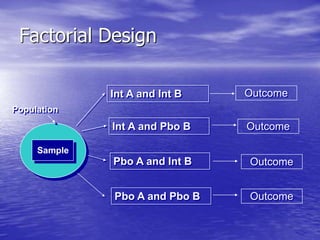
This document provides an overview of different study designs including observational and experimental designs. It defines key observational designs like case reports, case series, ecological studies, cross-sectional studies, case-control studies and cohort studies. It notes their strengths and weaknesses. Experimental designs discussed include randomized controlled trials and their key elements like selection of subjects, allocation of exposure, blinding and analysis. Clinical trial phases and ethical principles in trials are also summarized.