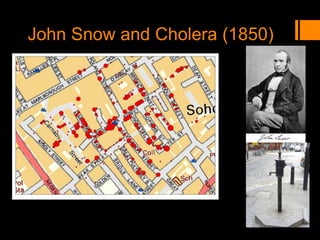
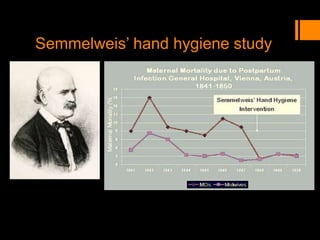
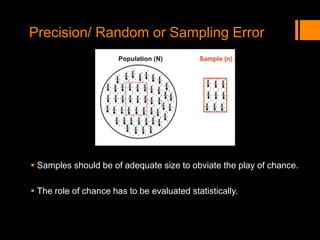
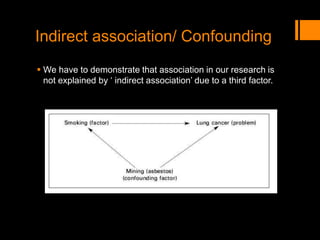
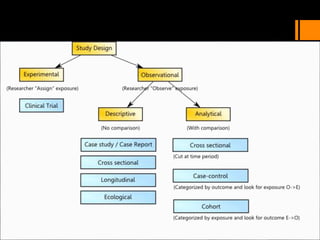
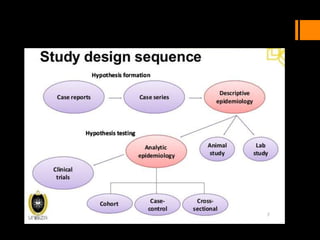
This document discusses the history and importance of clinical research. It notes that while medical research has only recently emerged as a formal discipline, epidemiological practices date back centuries to figures like Hippocrates, James Lind, Edward Jenner, John Snow, Ignaz Semmelweis, and Joseph Goldberger. Their studies helped establish strong methodologies in the 1940s-1950s. The document outlines reasons for conducting research, including fulfilling degree requirements, advancing medical knowledge as the field continues expanding, and contributing to the art and science of medicine. It argues doctors should be trained in research to apply findings wisely, produce research that helps colleagues, and consume research accurately to treat patients. Finally, it describes seven key reasons related to