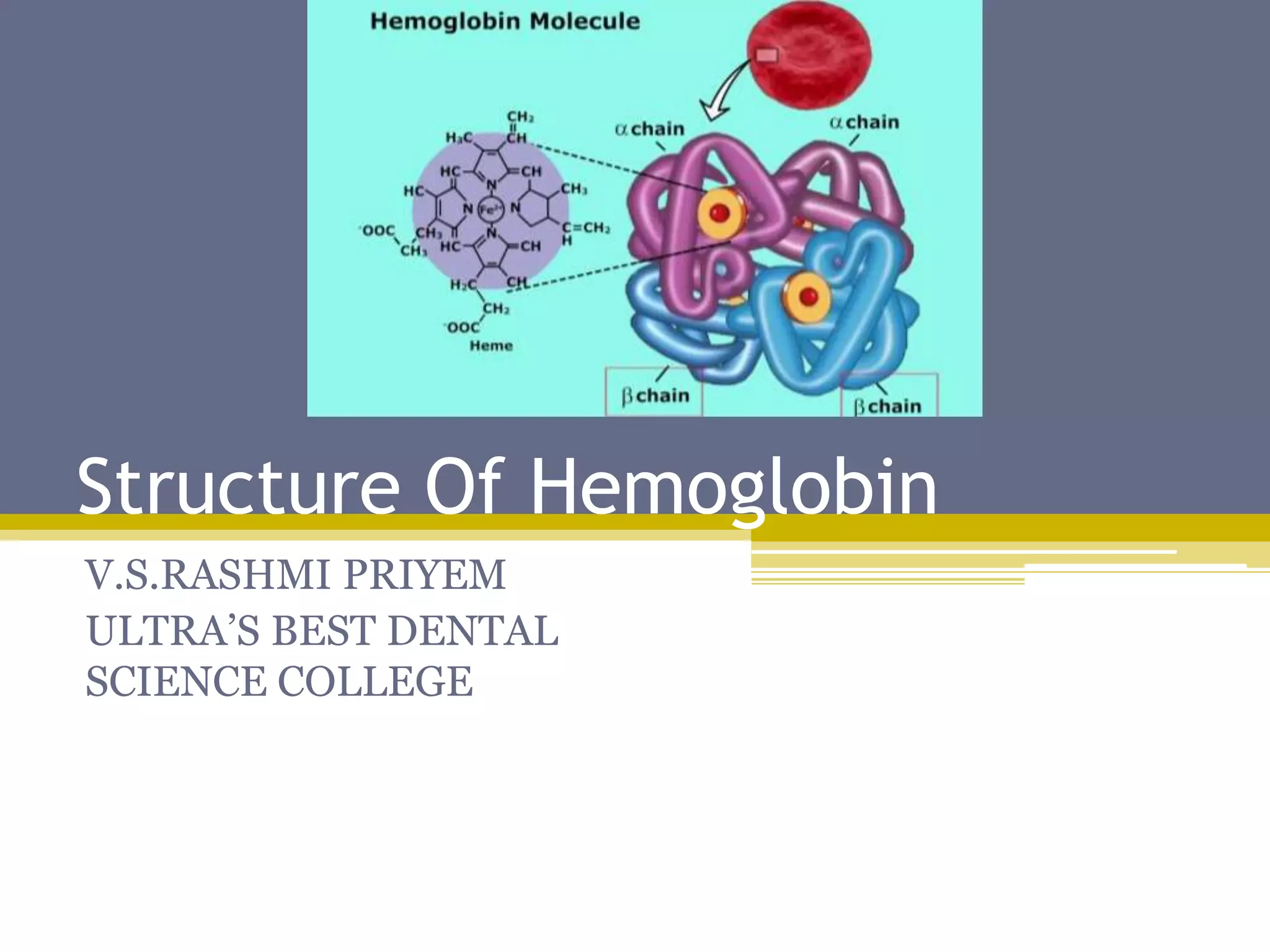
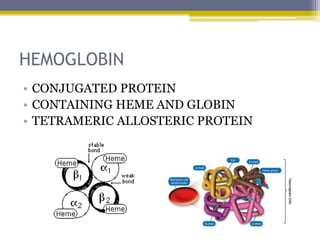
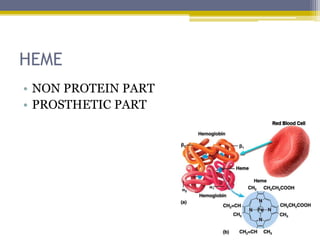
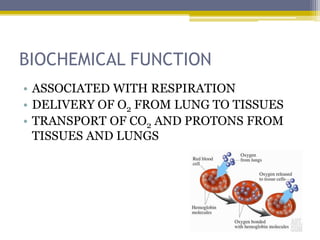
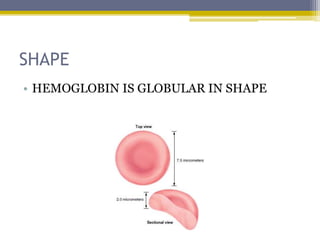
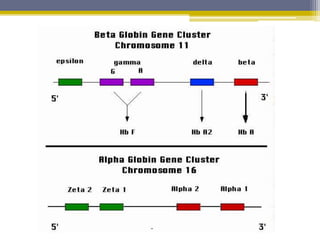
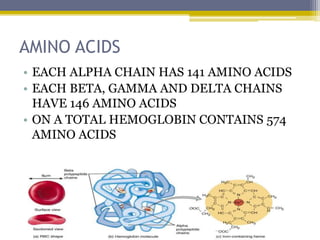
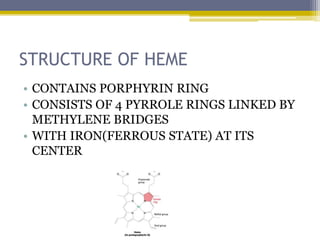
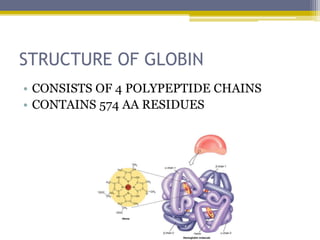
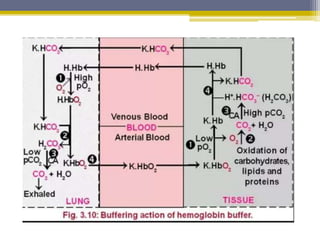
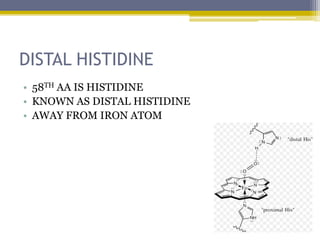
Hemoglobin is a conjugated protein composed of heme and globin subunits. It is a tetrameric allosteric protein that transports oxygen from the lungs to tissues and carbon dioxide from tissues back to the lungs. Hemoglobin exists as HbA, HbA2, and HbF, which are composed of different combinations of alpha, beta, gamma, and delta globin chains. It has a globular shape and contains heme at its center, which non-covalently binds to the globin protein through hydrophobic, ionic, and hydrogen bonding interactions.