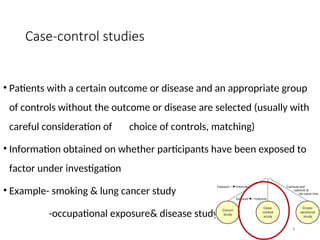
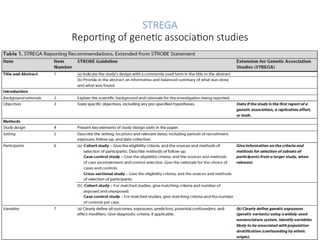
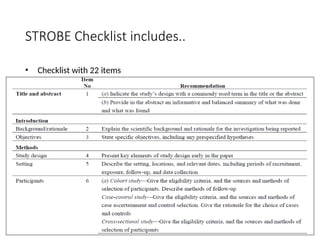
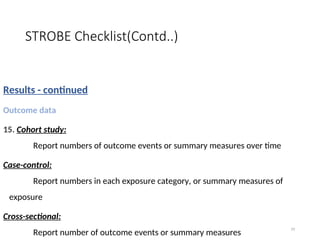
The document presents the STROBE checklist, a widely utilized tool for the transparent reporting of observational studies in epidemiology, developed in 2004. It includes a 22-item checklist focusing on cohort, case-control, and cross-sectional study designs, as well as extensions like STR EGA for genetic association studies and STROBE-ME for molecular epidemiology. The checklist ensures thoroughness in study reporting to enhance reproducibility and interpretation of findings.