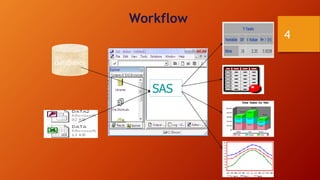
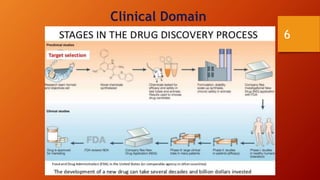
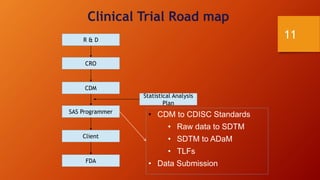
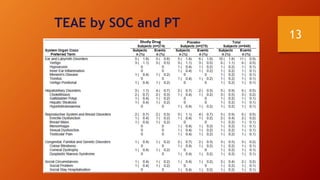
SAS is a statistical analysis software primarily used as a data management, statistical analysis, and reporting tool. It was created at North Carolina State University in the late 1960s. The typical SAS workflow involves collecting data from databases, performing analysis and reporting in SAS, and submitting clinical trial data and results to regulatory agencies like the FDA. SAS plays a key role in organizing clinical trial data into CDISC standards like SDTM and ADaM and generating tables, figures, and listings for analysis and regulatory submission.