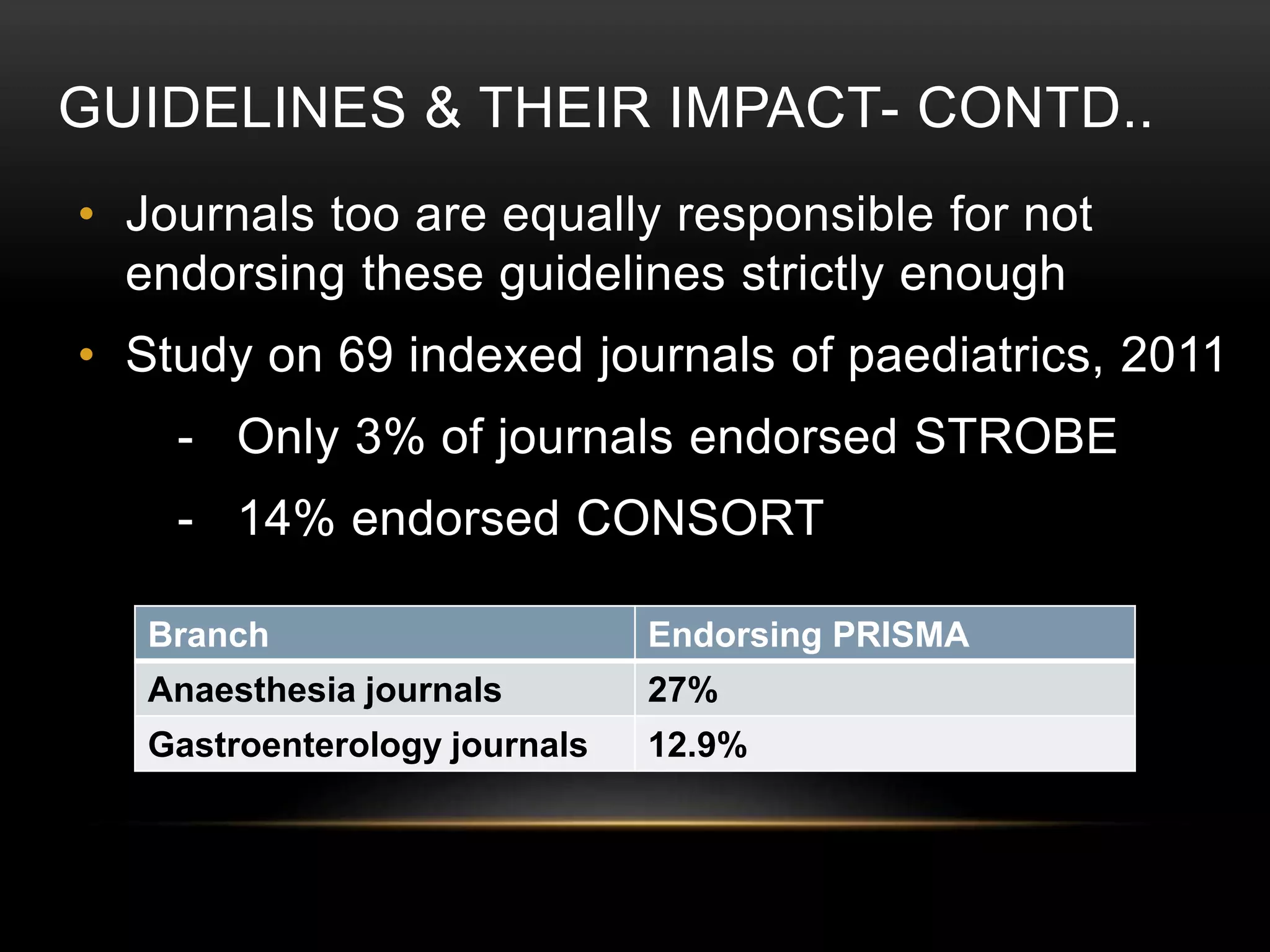
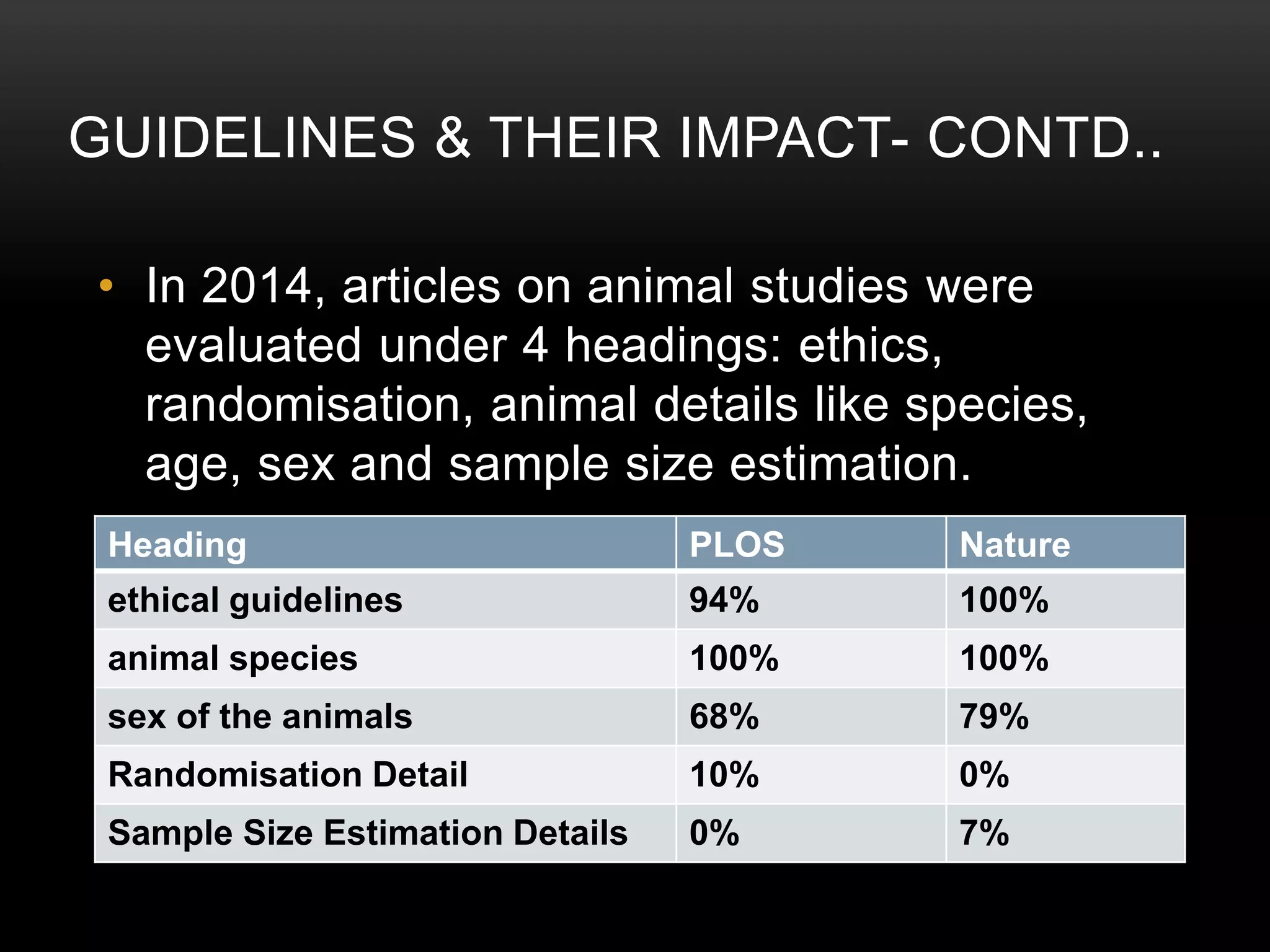
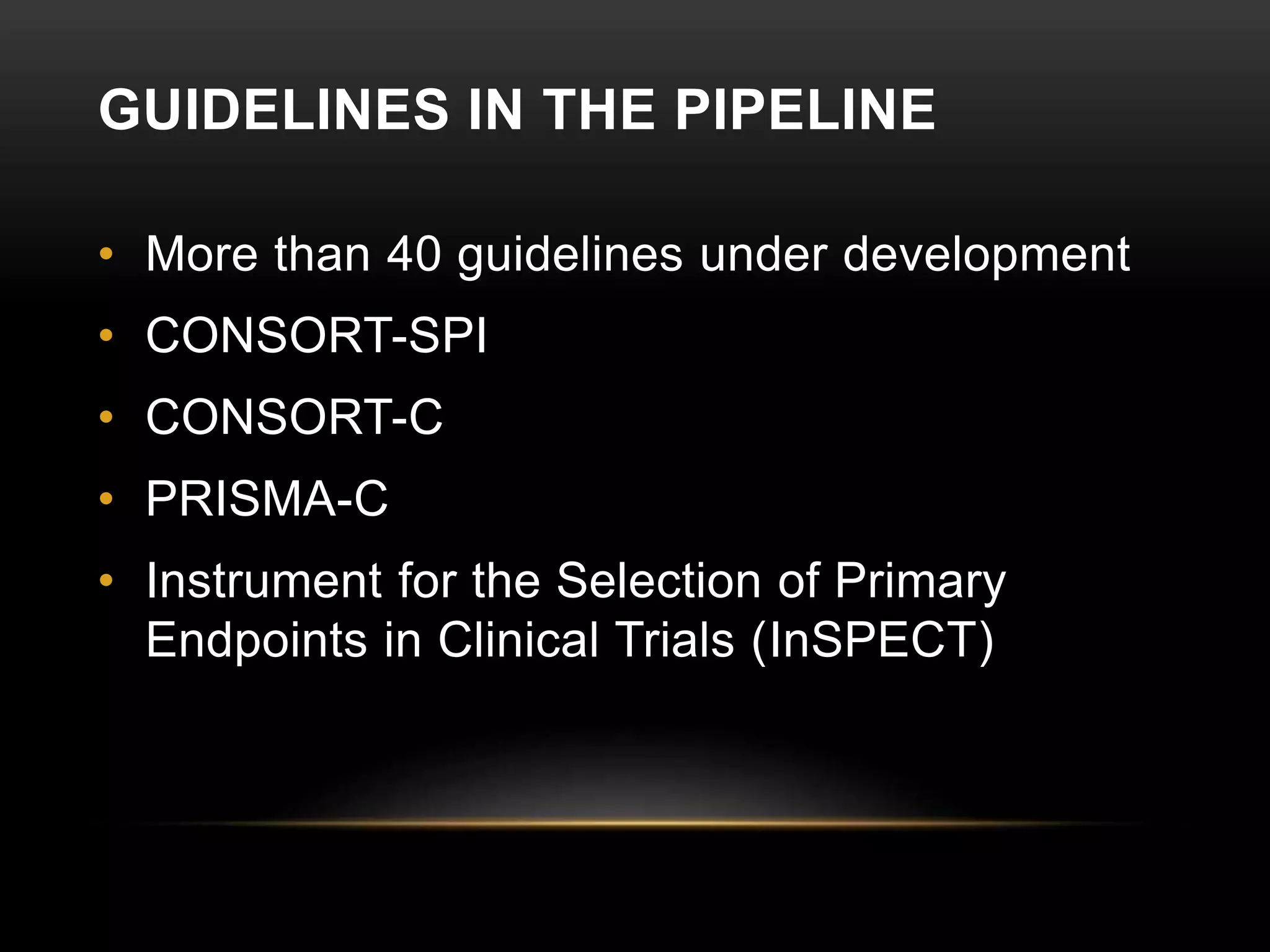
This document provides an overview of various clinical trial reporting guidelines developed by the EQUATOR Network, including CONSORT for randomized controlled trials, STROBE for observational studies, PRISMA for systematic reviews/meta-analyses, and ARRIVE and CARE for animal and case studies respectively. It discusses the goals of the EQUATOR Network, describes the development and components of these guidelines, and reviews evidence on their impact in improving the quality and transparency of research reporting over time, though adoption remains incomplete.