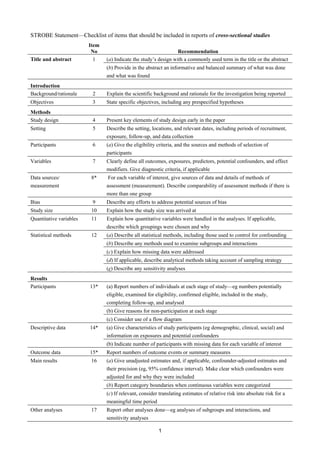
This document outlines 22 checklist items that should be included when reporting the results of cross-sectional studies. The checklist includes recommendations for the title, abstract, introduction, methods, results, discussion, and other sections of reports. Key elements that should be reported include the study design, setting, participants, variables, data sources, statistical methods, numbers of participants, results, limitations, interpretation, and generalizability. Providing this information transparently allows readers to understand what was done in the study and determine if the results are valid.