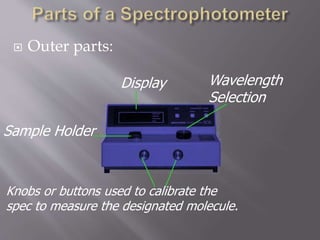
This document discusses different methods for detecting molecules in solutions, including indicator solutions and spectrophotometers. It describes how spectrophotometers work by shining light on a sample and measuring how it absorbs, reflects, or transmits the light. Spectrophotometers can use ultraviolet or visible light and measure transmittance, converting it to absorbance using Beer's Law to determine concentrations of unknown samples. The document provides details on the inner parts of spectrophotometers including lamps, prisms or gratings, and outer parts like sample holders and displays.