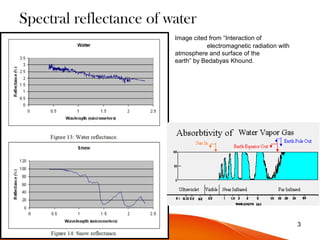
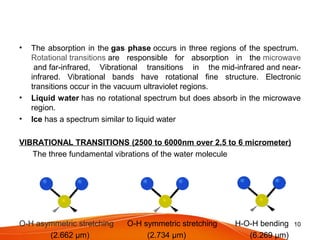
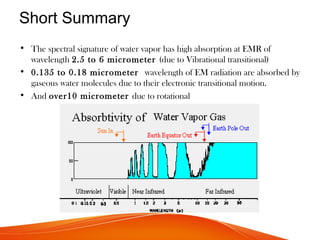
The document discusses the spectral signature of water, emphasizing how its absorption and reflectance of electromagnetic radiation vary with wavelength, influenced by its physical state. Key concepts include the Beer-Lambert law, which relates attenuation of light to material properties, and the significant absorption characteristics of water in different forms (liquid, vapor, ice) within specific wavelength ranges. Overall, it highlights the scientific basis for distinguishing water types and analyzing their spectral responses.


![Background on spectral signatures
• For any given material, the amount of solar radiation that is reflected
(absorbed, transmitted) will vary with wavelength. This important property of
matter allows us to separate distinct cover types based on their response
values for a given wavelength. When we plot the response characteristics of
a certain cover type against wavelength, we define what is termed the
spectral signature of that cover. The diagram below illustrates the spectral
signatures for some common cover types. [2]
4
Figure: Remote Sensing #2: Spectral Signatures by Ryan Swearingen](https://image.slidesharecdn.com/spectralsignature-150122215215-conversion-gate01/85/Spectral-signatures-4-320.jpg)

![Quantifying the problem
• The absorbance of an object
quantifies how much of the
incident light is absorbed by it
(instead of being reflected or
refracted).
• Beer-Lambert Law[3]
– It relates the attenuation of light to
the properties of the material
through which the light is traveling.
• "The absorption is directly proportional
to the thickness of the object or in other
words the transmittance is inversely
proportional to the path length travelled
by the radiation into the bulk"
Physical Quantities in
Formula:
T Transmissivity
Σ attenuation coefficient
ℓ distance of light
travelled through material
ε absorptivity
c concentration of
material
Io & I Intensity of incident &
transmitted radiation](https://image.slidesharecdn.com/spectralsignature-150122215215-conversion-gate01/85/Spectral-signatures-6-320.jpg)
![Beer–Lambert law in the atmosphere[3]
'Tx refers to the optical depth travelled by radiation in x matter (absorption or
scattering'
Ta refers to aerosols (that absorb and scatter)
Tg are uniformly mixed gases (mainly carbon dioxide (CO2) and molecular
oxygen (O2) which only absorb)
TRS are effects due to Raman scattering in the atmosphere
TNO2 is nitrogen dioxide, mainly due to urban pollution (absorption only)
Tw is water vapour absorption
TO3 is ozone (absorption only)
Tr is Rayleigh scattering from molecular oxygen (O2) and nitrogen (N2)
(responsible for the blue color of the sky).
Did above formula reminded you this ... ?
gb = gobs - gr + ( gL + gFA + gB + gT)
"Bouguer Gravity Anomaly"](https://image.slidesharecdn.com/spectralsignature-150122215215-conversion-gate01/85/Spectral-signatures-7-320.jpg)
![Dependence of absorption on the physical state
• In some fluids the absorption depends on their physical state too.
The absorption of electromagnetic radiation by water depends on
it’s state. [1]
8
Figure:
Absorption spectrum
(attenuation coefficient(Yaxis)
vs. wavelength-Xaxis) of
liquid water (red), water vapor
(green) and ice (blue line)
between 667 nm and 200μm.
The plot for vapor is a
transformation of data Syn
-thetic spectrum for gas
mixture 'Pure H2O' (296K,
1 atm)](https://image.slidesharecdn.com/spectralsignature-150122215215-conversion-gate01/85/Spectral-signatures-8-320.jpg)
![Fig. EMR Spectral regions [1]](https://image.slidesharecdn.com/spectralsignature-150122215215-conversion-gate01/85/Spectral-signatures-9-320.jpg)

![Reference
[1] Wikipedia article: Electromagnetic Absorption by water, Link:
http://en.wikipedia.org/wiki/Electromagnetic_absorption_by_water
[2] Swearingen R., “Remote Sensing #2: Spectral Signature” Link:
www.iupui.edu/~ghw/lessons/materials/SpectralSig.doc.
[3] Wikipedia article: Beer-Lambert Law Link: "http://en.wikipedia.org/wiki/Beer
%E2%80%93Lambert_law"
13](https://image.slidesharecdn.com/spectralsignature-150122215215-conversion-gate01/85/Spectral-signatures-13-320.jpg)
