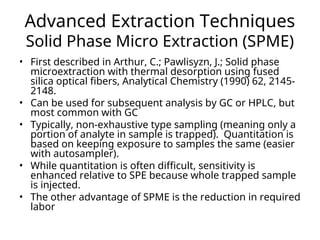
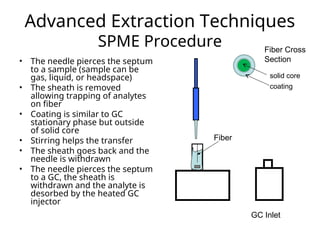
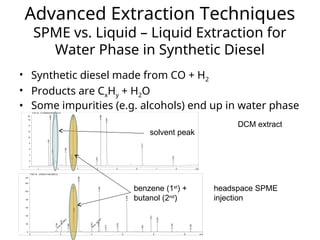
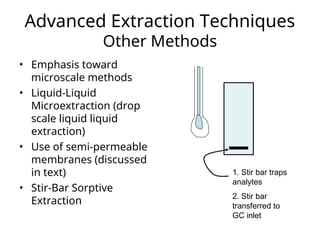
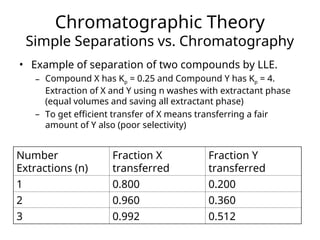
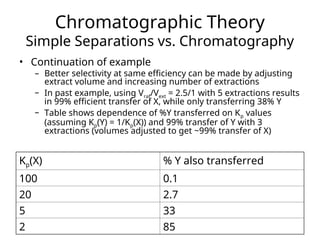
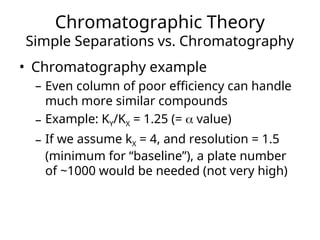
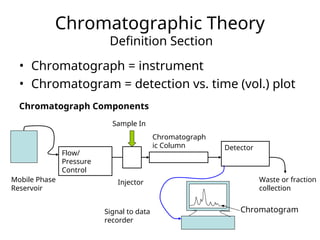
The document outlines the content of a chemistry lecture, focusing on solid phase microextraction (SPME) techniques and their applications in analysis, particularly in relation to gas and liquid chromatography. It covers the advantages and disadvantages of SPME compared to traditional extraction methods, as well as various advanced extraction techniques and chromatographic theory. The lecture also includes information on the upcoming exam and resources available on the course website.