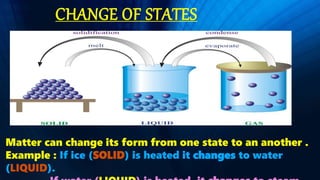
This document discusses the three states of matter - solids, liquids, and gases. It explains that in solids, particles are packed closely together, in liquids they are less closely packed, and in gases they are very loosely packed. It describes how matter can change states through melting, evaporation, condensation, and solidification. It also defines solutions as formed when substances mix evenly, with the substance present in lower amount called the solute and the substance it dissolves in called the solvent. Finally, it discusses different methods of separating substances including sedimentation and decantation, filtration, and evaporation.