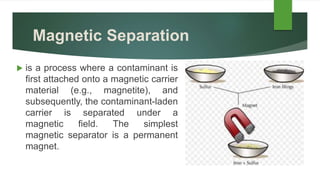
This document discusses the properties of matter and different types of matter. It defines chemistry as the study of matter and its composition, structure and properties. Matter is anything that has mass and takes up space. There are three main states of matter: solids, liquids, and gases. Pure substances like elements and compounds have a definite composition, while mixtures are combinations of two or more substances mixed together physically. The document outlines several methods for separating mixtures, such as filtration, decantation, centrifugation, evaporation and chromatography.