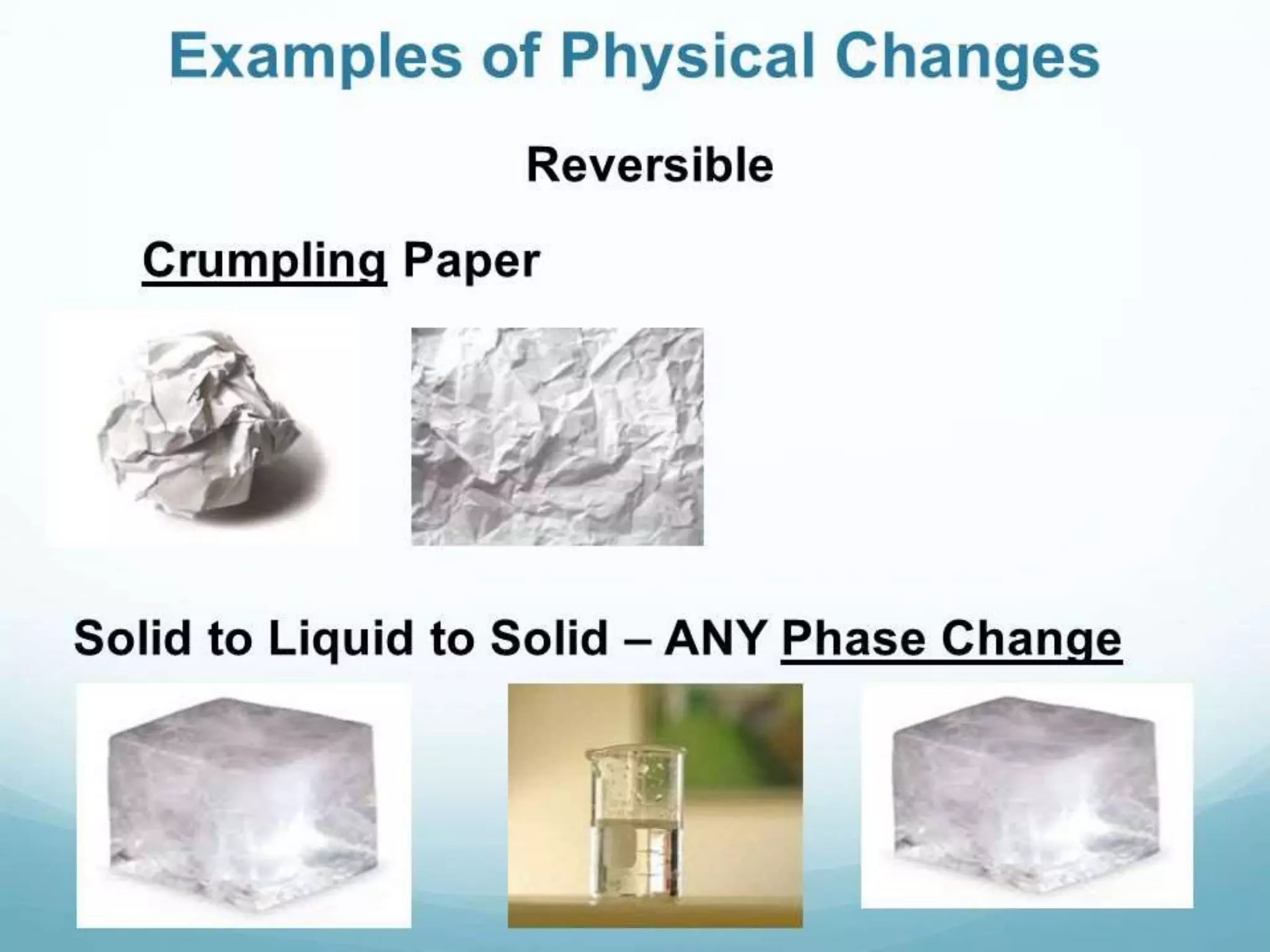
This document discusses physical and chemical changes. It defines change as something becoming different or taking another form. Physical changes alter a substance's physical properties but do not form new substances, while chemical changes produce new substances through chemical reactions. An example of a chemical change given is rusting of iron, which occurs through a reaction of iron with air and water to form iron oxide. The document also describes ways to prevent rusting, such as through galvanization which deposits a zinc layer on iron, and alloying iron with corrosion-resistant metals like in stainless steel.