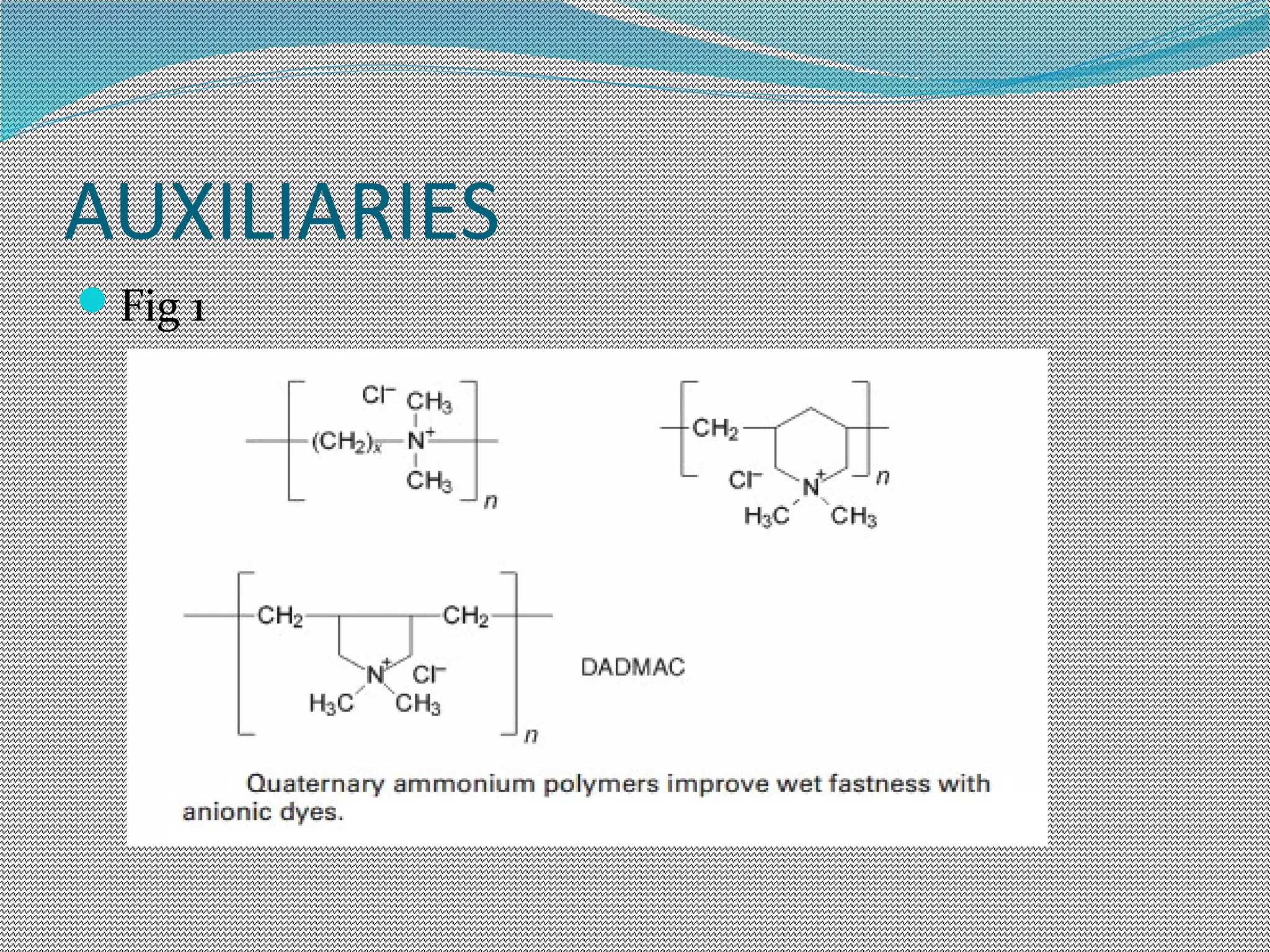
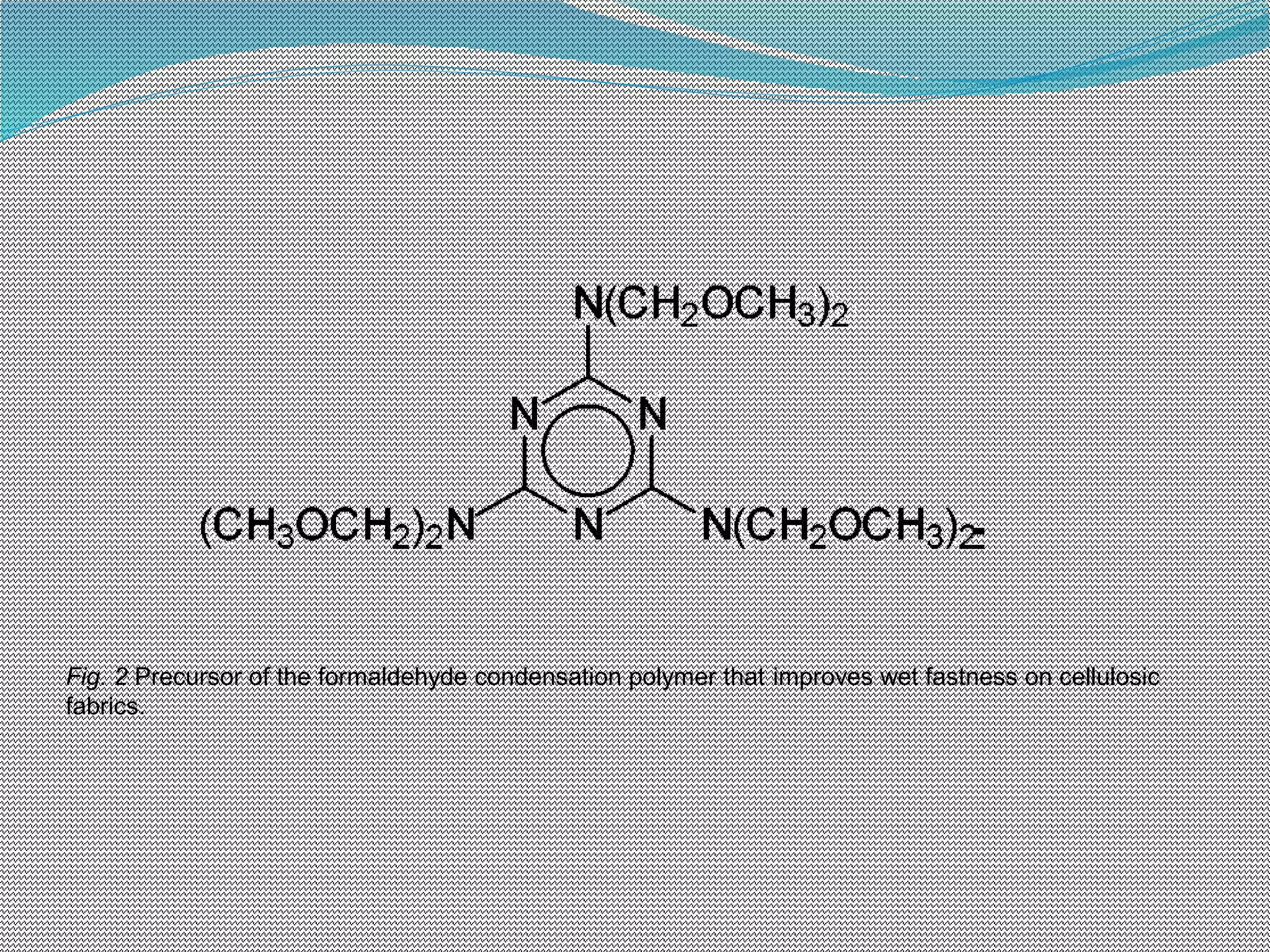
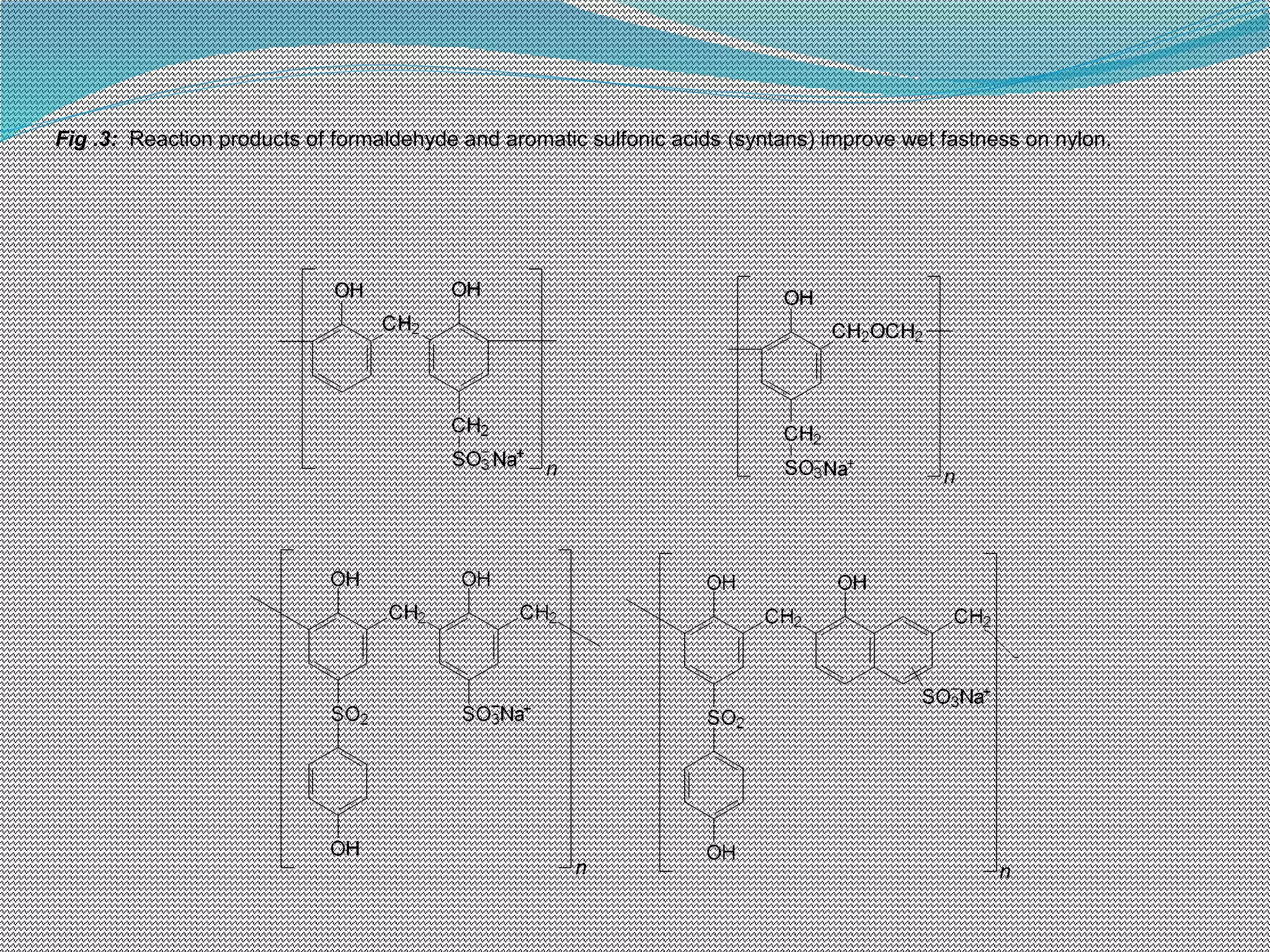
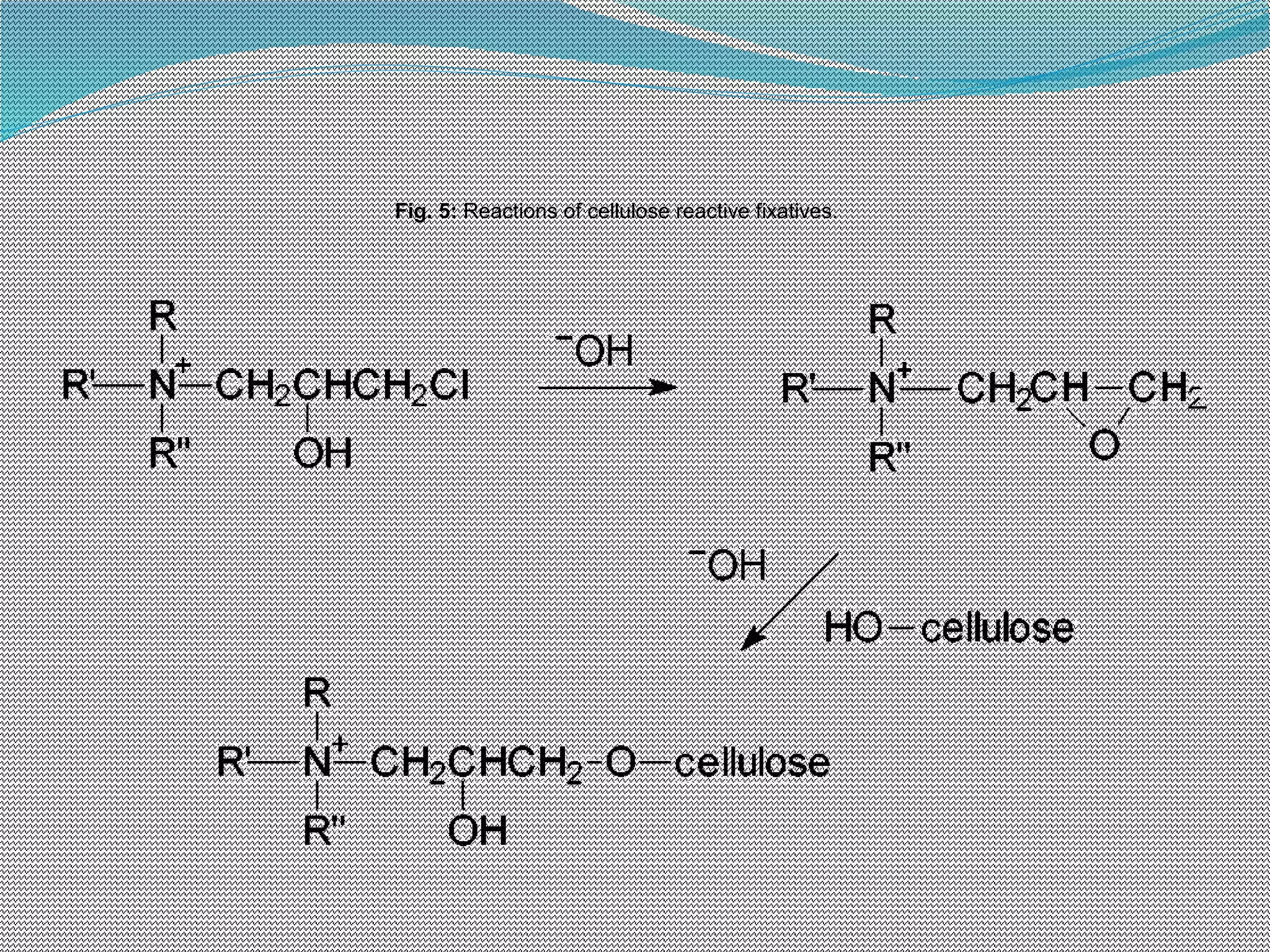
This document discusses various chemical finishing methods to improve the wet fastness of dyed textiles. It begins by defining key terms like colour fastness, fading, and bleeding. It then describes the ratings system used to evaluate fastness from 1-5 or 1-8. The rest of the document discusses specific approaches to improving wet fastness for cellulosic, synthetic, and nylon textiles using techniques like intensive washing, reductive agents, cationic products, formaldehyde condensation products, and reactive compounds. It also notes some potential issues with these methods and describes evaluation standards.