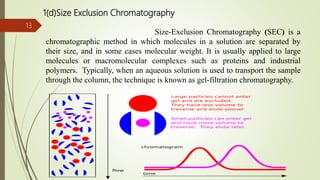
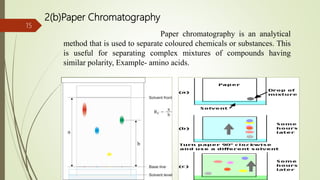
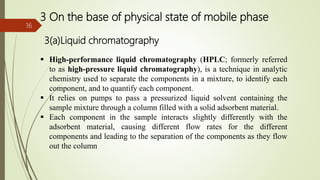
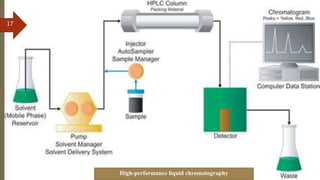
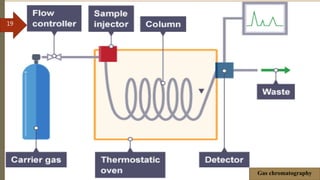
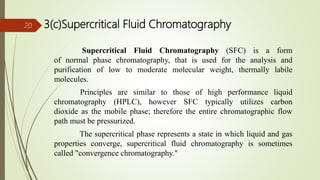
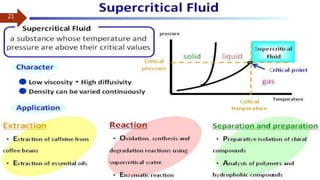
This document provides an overview of chromatography. It begins with an introduction that defines chromatography and describes how it separates mixtures based on differences in solubility between components in mobile and stationary phases. The document then covers the history of chromatography, important technical terms, main types categorized by interaction with the stationary phase or physical state of the mobile phase. Applications are discussed in areas like drug development, food testing, and forensics. Advantages are noted as versatility in separation. Disadvantages include temperature sensitivity and ensuring solubility. References are listed at the end.