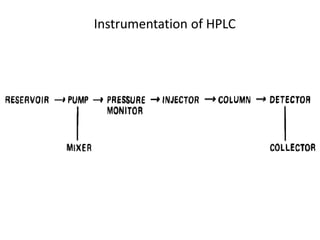
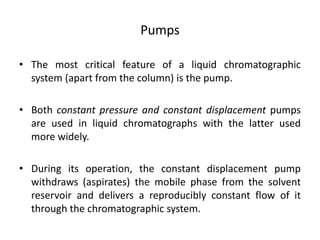
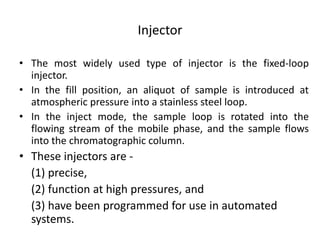
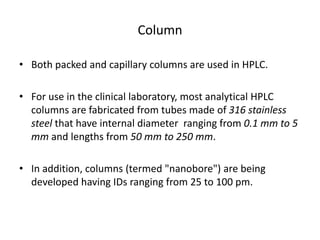
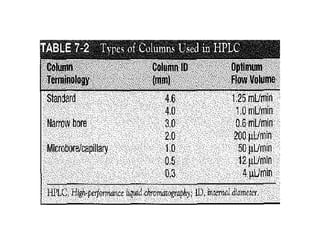
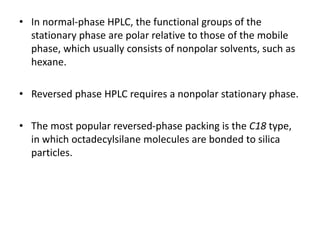
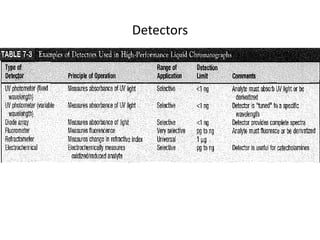
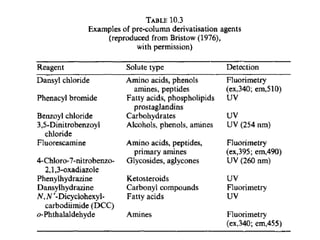
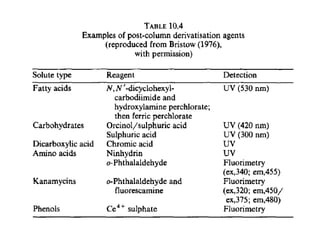
This document provides an overview of high-performance liquid chromatography (HPLC) and its use in pathology. It describes the basic components and separation mechanisms of chromatography, including mobile phase, stationary phase, and how separation occurs. It then discusses various types of chromatography techniques like ion exchange, partition, size exclusion, and affinity chromatography. The document focuses on HPLC, describing its instrumentation including reservoirs, pumps, injectors, columns, and detectors. It explains how HPLC provides improved resolution, speed, and reproducibility over other chromatography methods.





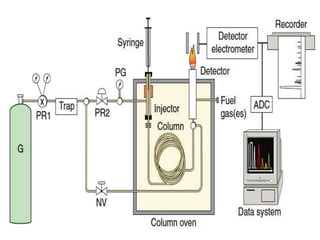
![Gas chromatography
• Gas chromatography (GC) is useful for compounds that are
naturally volatile or can be easily converted into a volatile
form.
• GC has been a widely used method for decades owing to its
high resolution, low detection limits, accuracy, and short
analytic time.
• Two types of stationary phases commonly used in GC are
solid absorbent (gas–solid chromatography [GSC]) and liquids
coated on solid supports (gas–liquid chromatography [GLC]).](https://image.slidesharecdn.com/maluseminarapril242017hplc-170902105103/85/HPLC-in-Pathology-20-320.jpg)