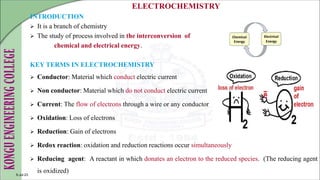
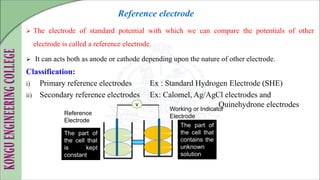
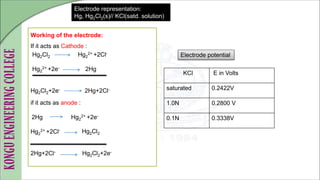
The document is a comprehensive overview of electrochemistry, detailing concepts such as cell types, electrode potentials, and the workings of reference electrodes like the standard hydrogen and calomel electrodes. It includes definitions of key terms, the construction and measurement techniques for electrodes, and describes the redox reactions involved in electrochemical processes. Practical applications and references to further literature are also provided.