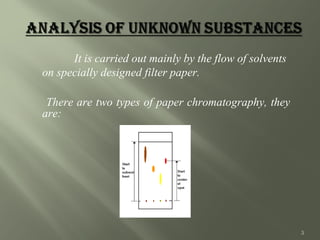
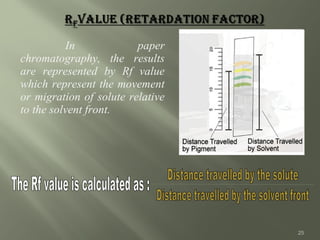
Paper chromatography (PC) was introduced in 1865 and is considered the simplest chromatographic technique. It involves using filter paper as the stationary phase and a solvent as the mobile phase. There are two types of PC - adsorption chromatography where the paper is coated with an adsorbent like silica or alumina, and partition chromatography where moisture in the paper acts as the stationary phase. Compounds are separated based on their partitioning between the stationary and mobile phases. PC is useful for separating mixtures and identifying compounds due to its low cost, simplicity, and good resolving power.