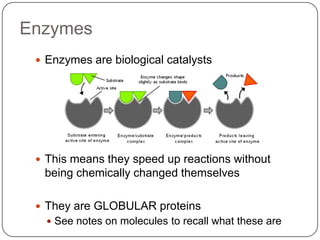
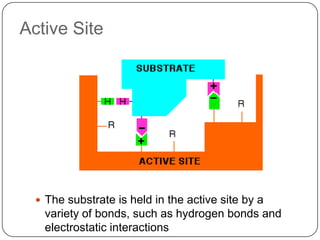
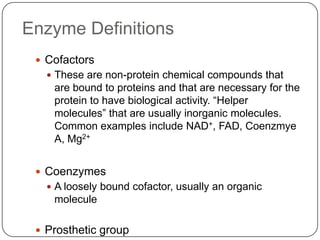
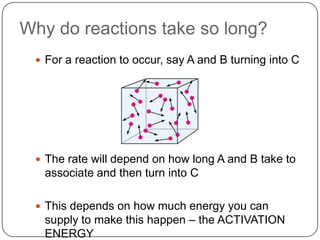
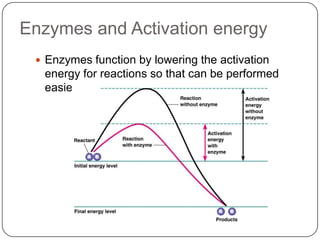
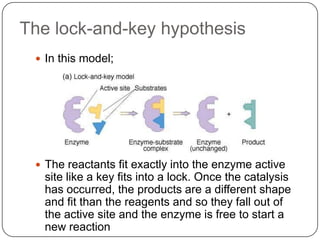
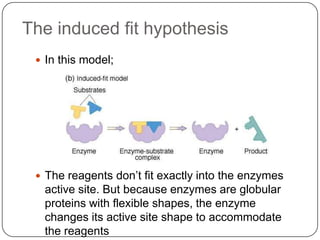
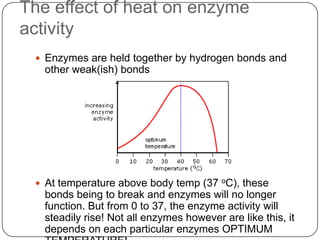
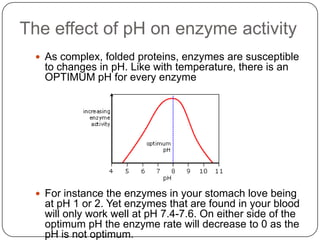
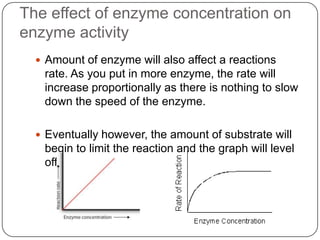
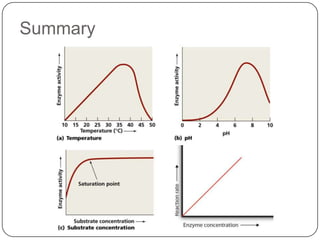
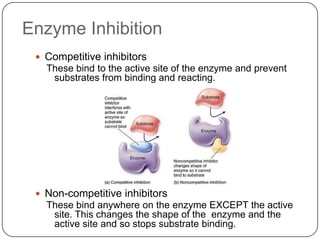
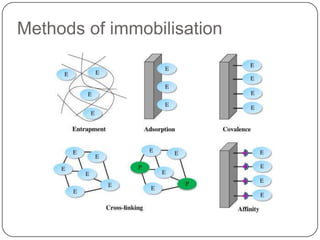
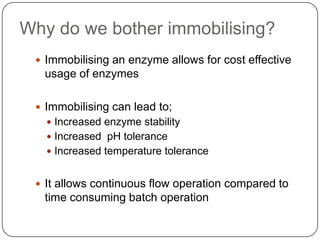
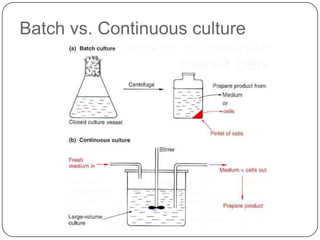
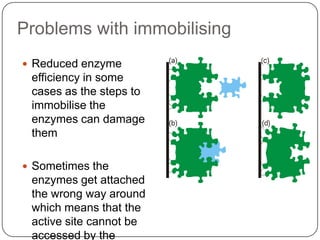
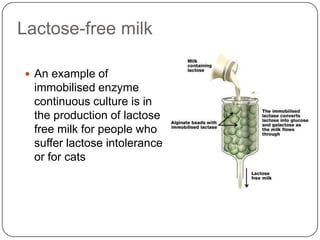
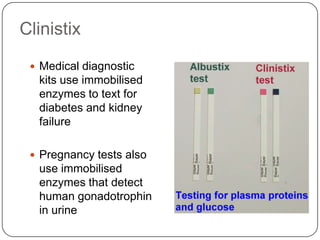
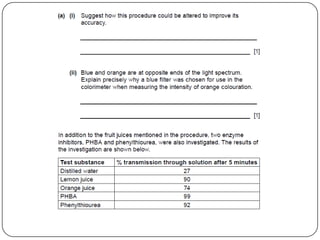
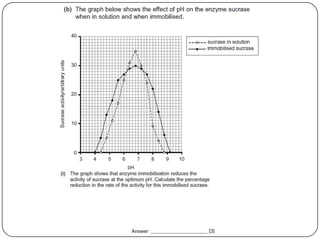
Enzymes are biological catalysts that speed up chemical reactions without being changed themselves. They are globular proteins that contain an active site where substrates bind and reactions occur. Enzymes lower the activation energy of reactions, allowing reactions to proceed more quickly. The lock-and-key and induced fit hypotheses describe how enzymes and substrates interact. An enzyme's activity can be affected by factors like temperature, pH, substrate and enzyme concentration. Enzymes can be immobilized to increase stability and allow continuous reaction processes. Common immobilization methods include cross-linking and adsorption.