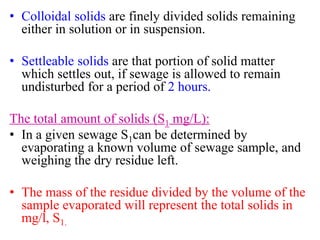
- The document describes the characterization of sewage based on its physical, chemical, and biological properties.
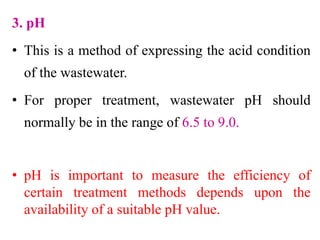
- Key physical characteristics include turbidity, color, odor, and temperature. Chemical characteristics include solids, alkalinity, pH, nitrogen compounds, and phosphorus. Biological characteristics involve bacteria and biochemical oxygen demand (BOD).
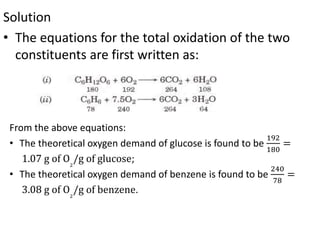
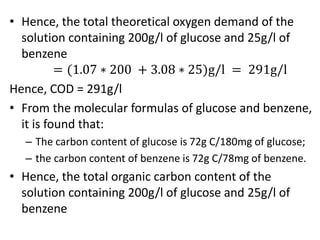
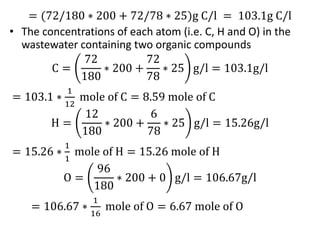
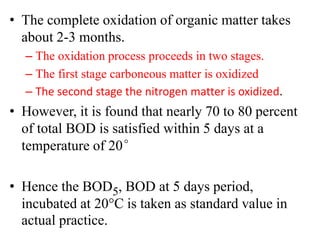
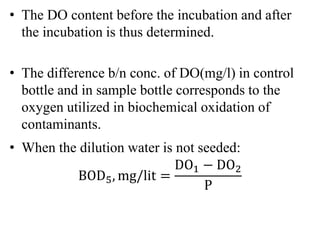
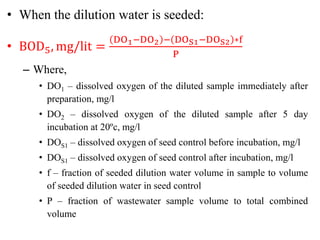
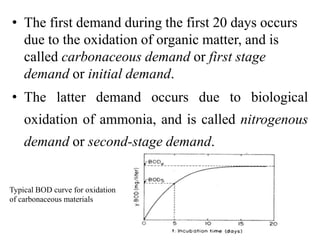
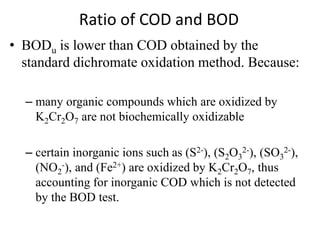
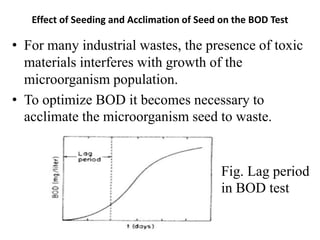
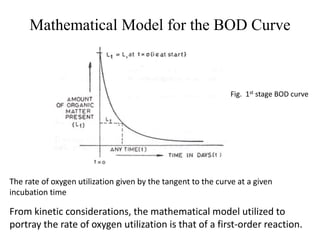
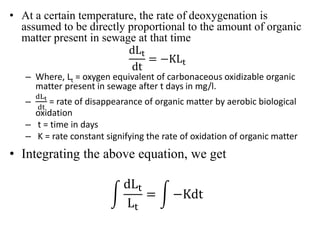
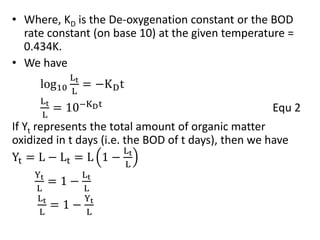
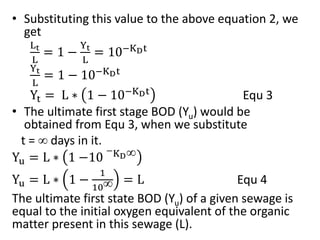
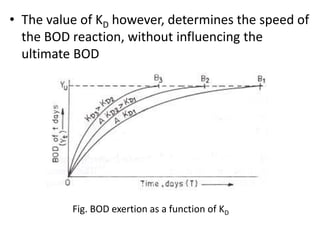
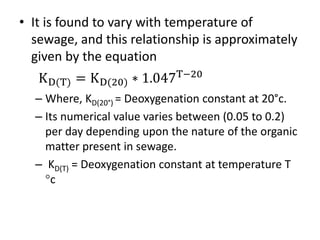
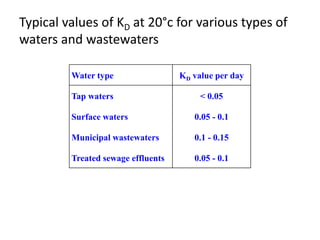
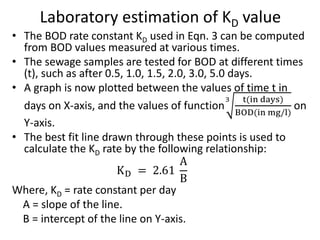
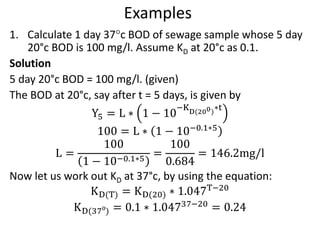
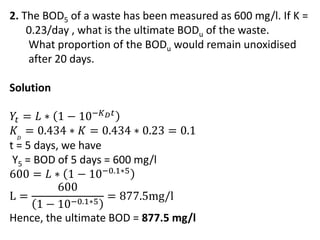
- BOD measures the amount of oxygen consumed by microorganisms to break down organic matter. It is used to assess water quality, with higher BOD indicating more pollution. The document provides equations to model BOD over time based on temperature and other factors.




















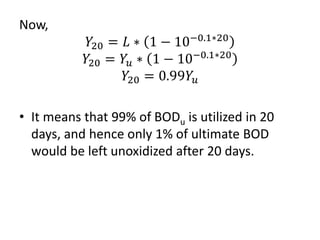


![• Experimentally:
– a known quantity of wastewater is mixed with a
known quantity of standard solution of potassium
dichromate
– the mixture is heated
– The organic matter is oxidized by K2Cr2O7 in the
presence of H2SO4
– The resulting solution of K2Cr2O7 is titrated with
standard ferrous ammonium sulphate
[Fe(NH4)2.(SO4)2.6H2O)],
– the oxygen used in oxidizing the wastewater is
determined.
– This is called the COD and is a measure of organic
matter present in sewage.](https://image.slidesharecdn.com/chapter2-231228132859-fa7436fa/85/Chapter-2-pptx-46-320.jpg)