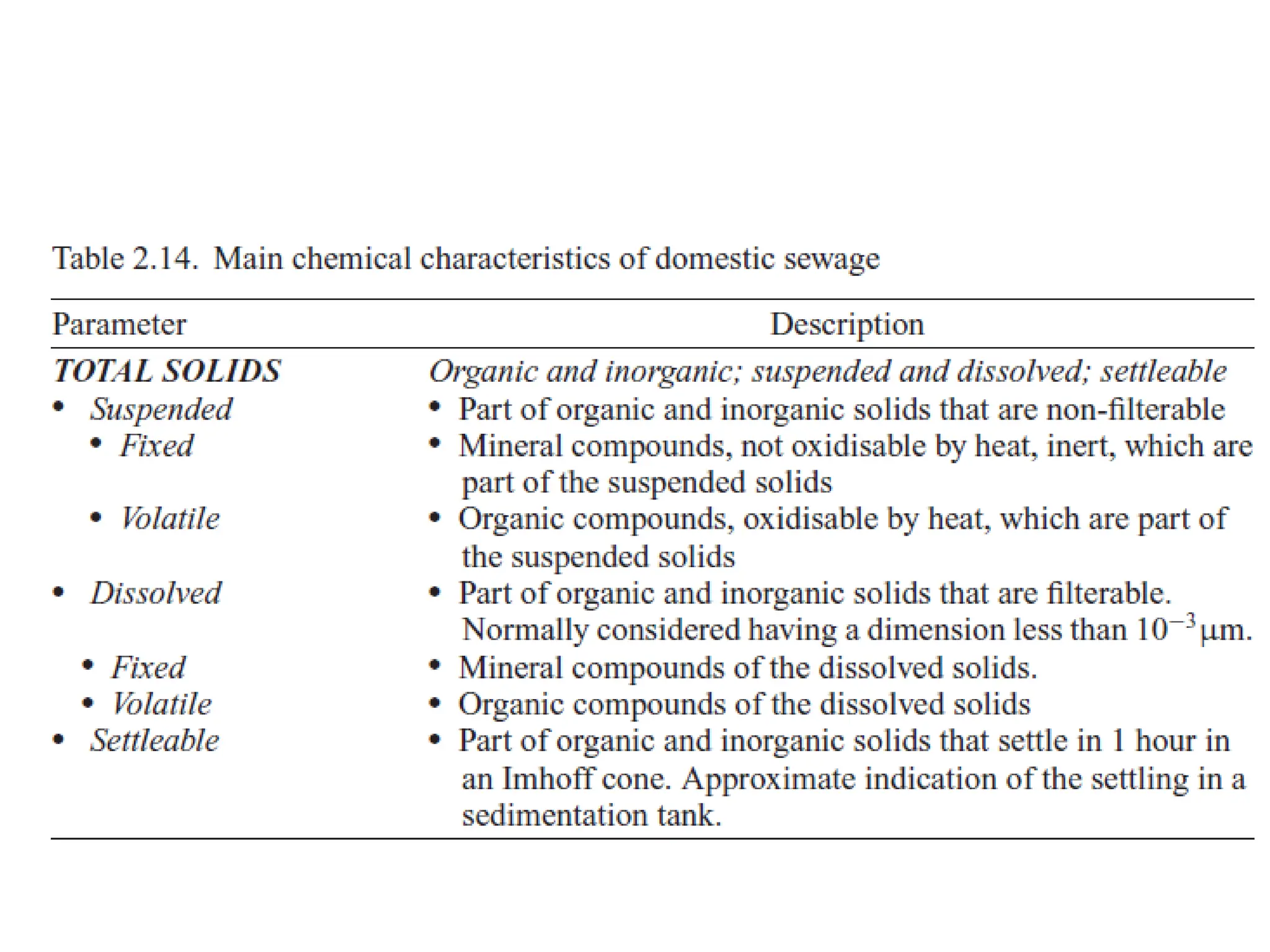
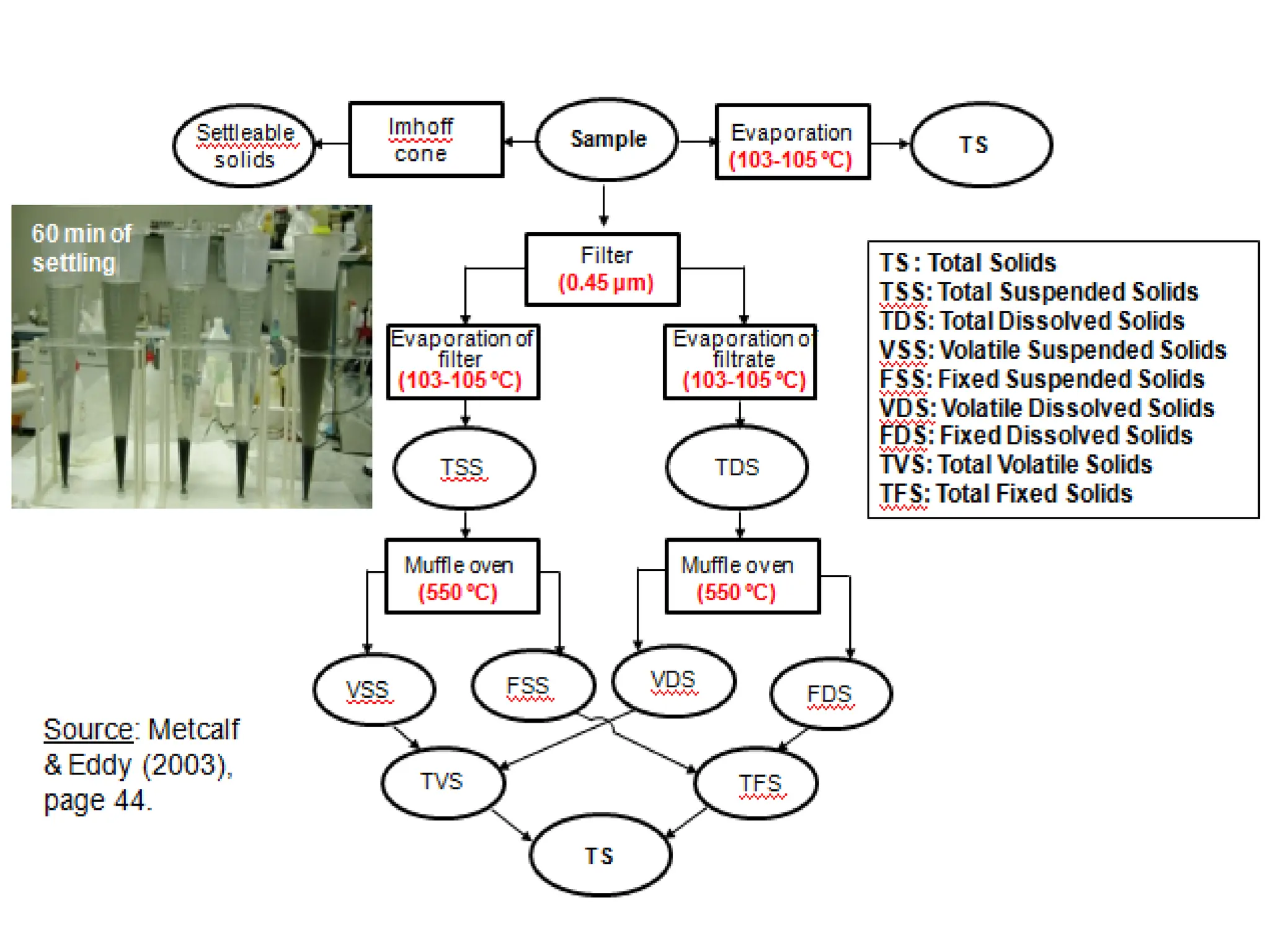
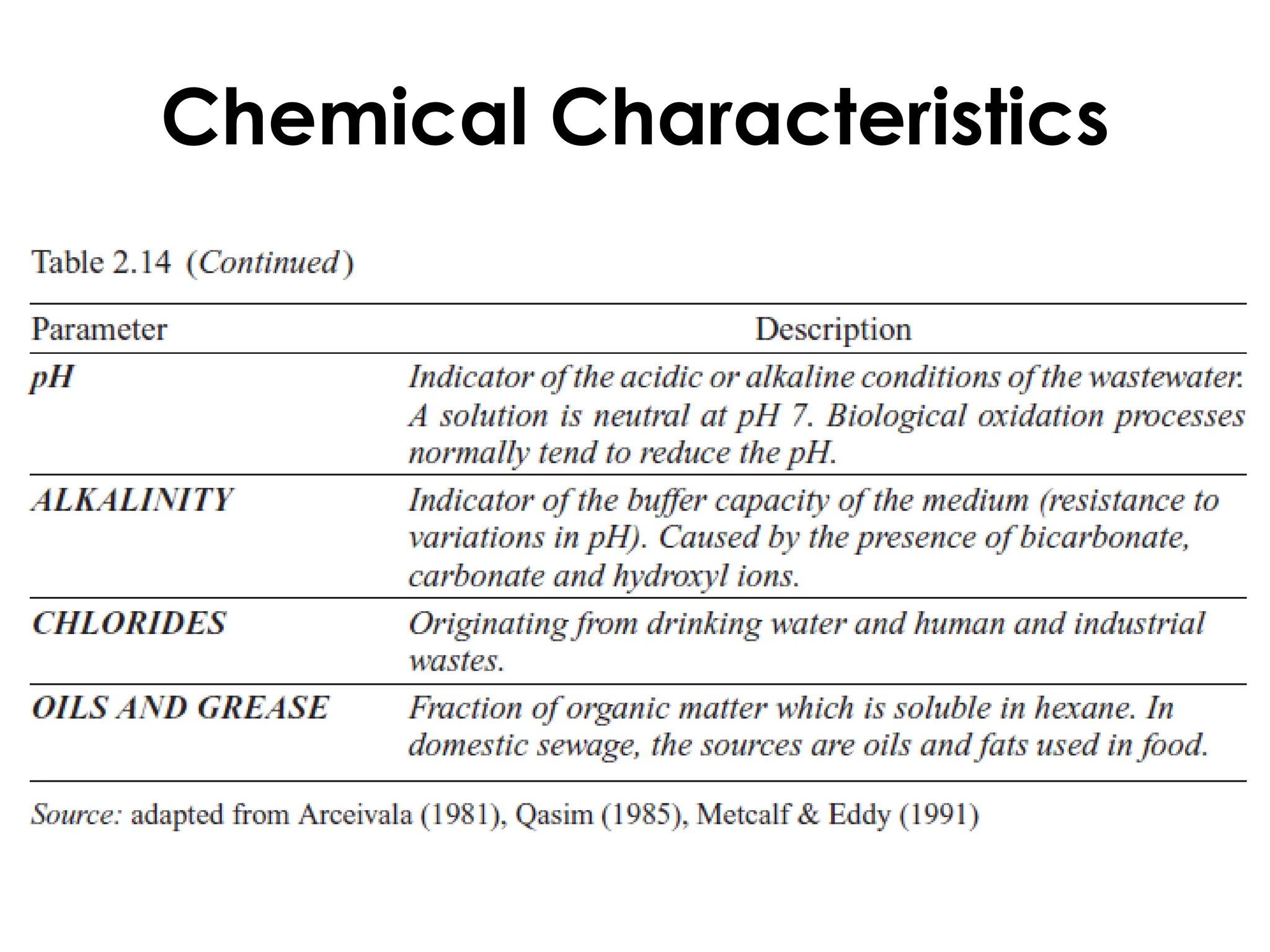
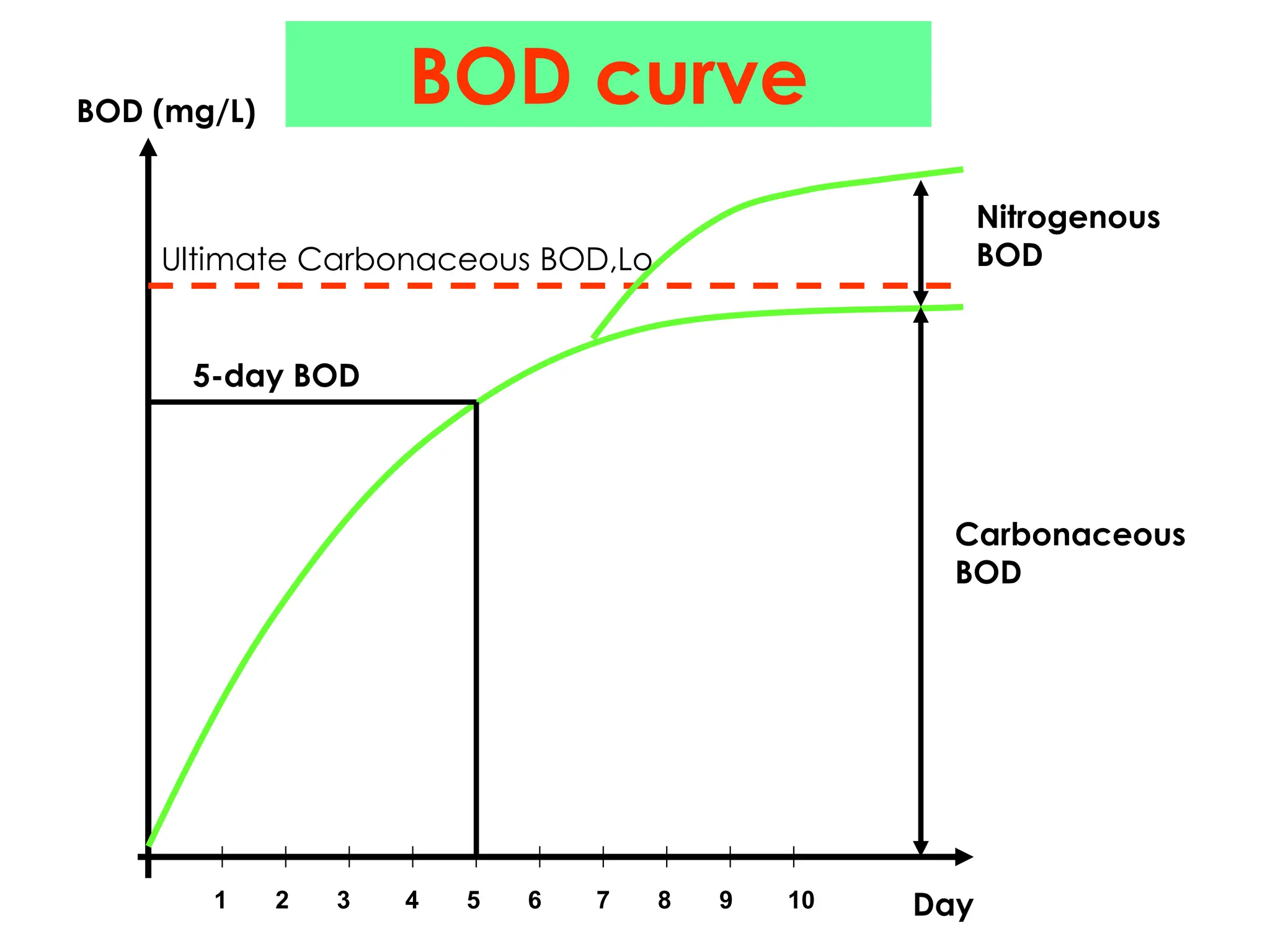
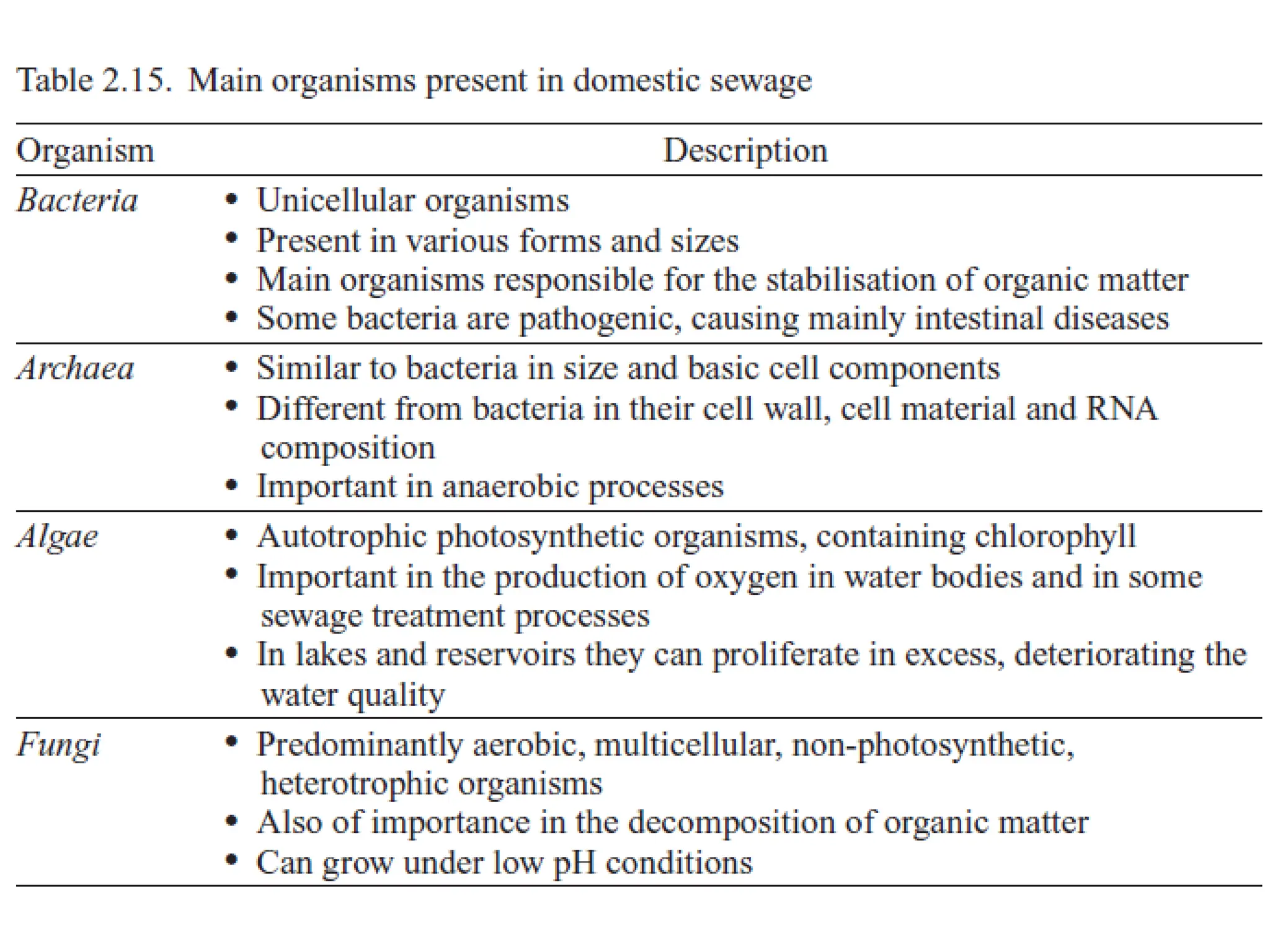
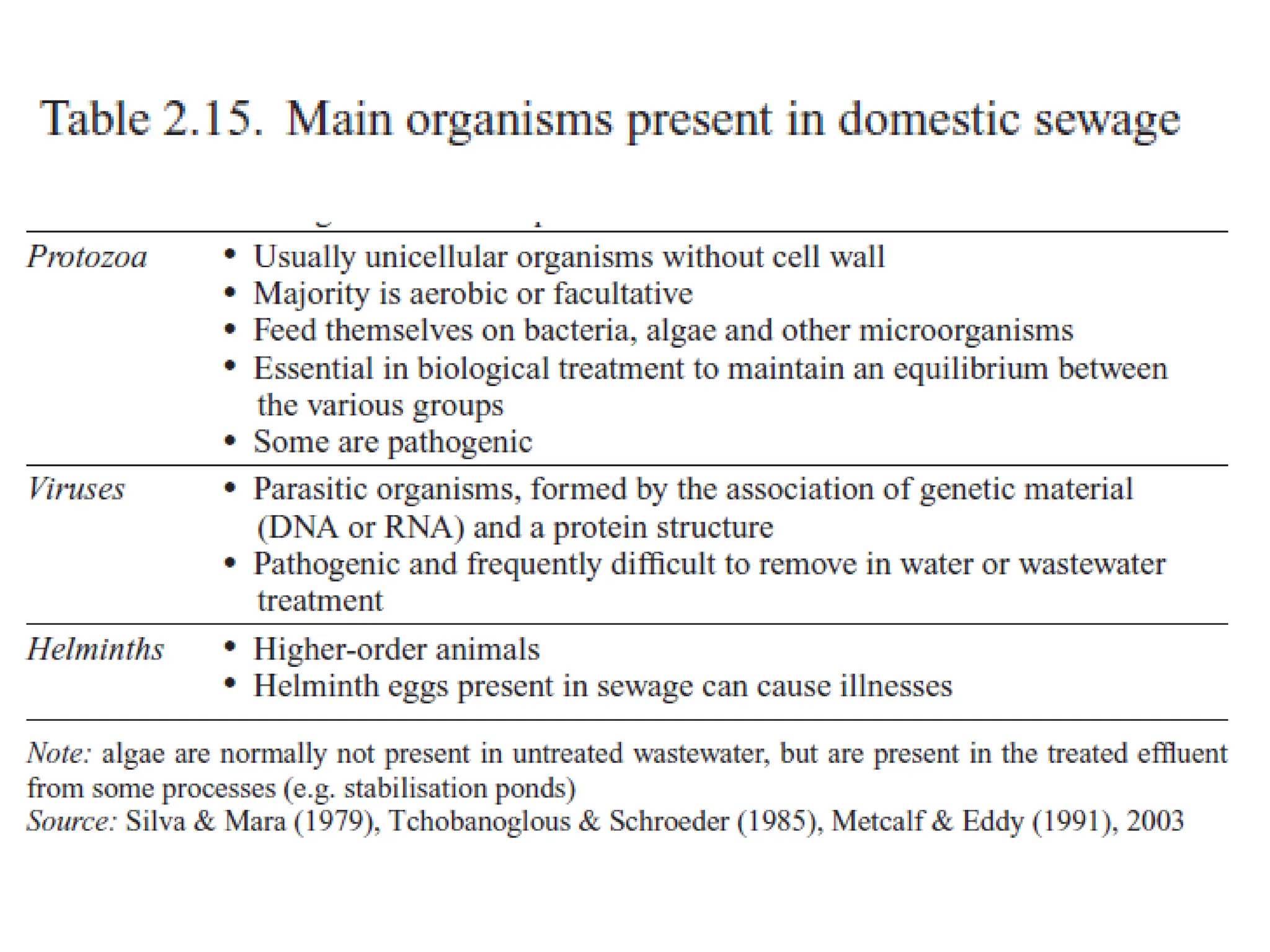
The document discusses wastewater types, sources, and characteristics, emphasizing the necessity of treatment to prevent health hazards and environmental pollution. It categorizes wastewater into domestic, industrial, and stormwater, while detailing physical, chemical, and biological characteristics, including methods to measure biochemical and chemical oxygen demand (BOD and COD). The text further explains the role of microorganisms in wastewater treatment and the significance of indicator bacteria for monitoring water quality.