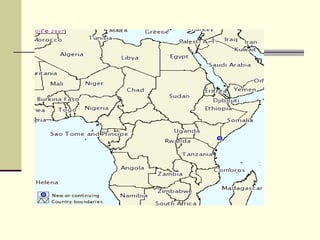
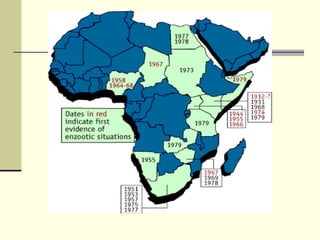
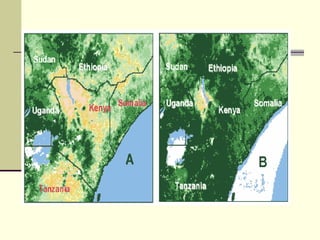
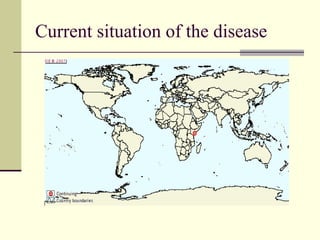
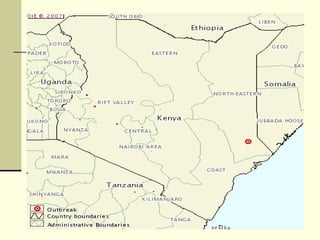
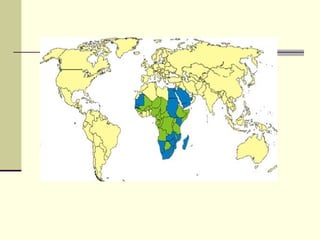
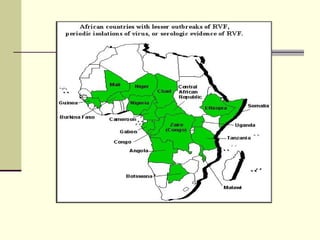
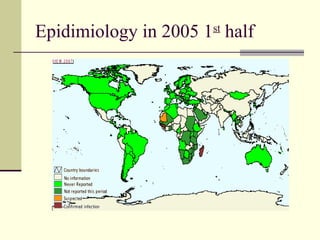
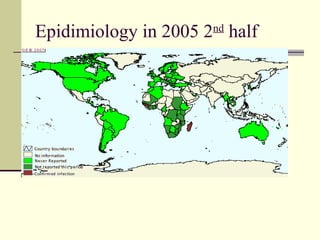
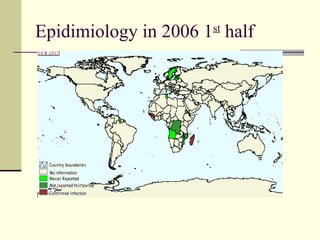
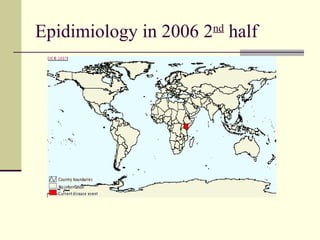
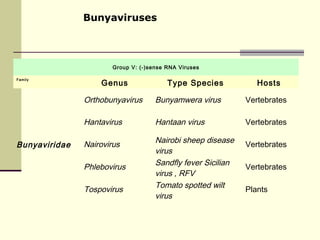
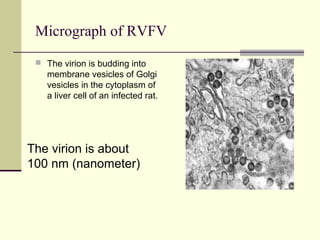
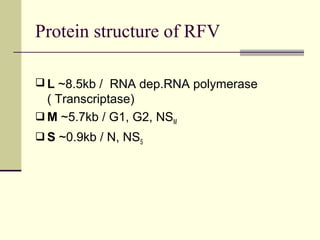
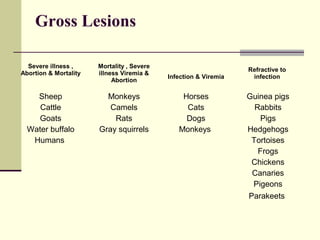
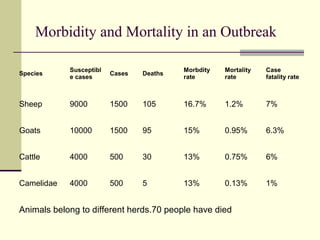
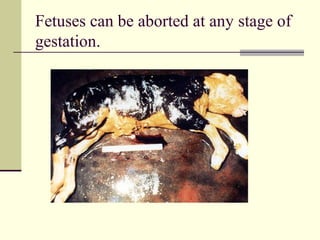
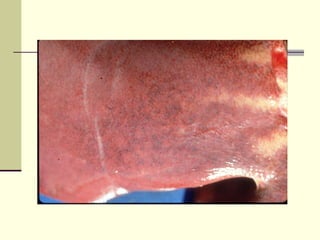
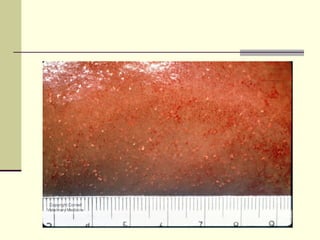
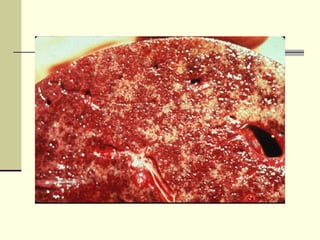
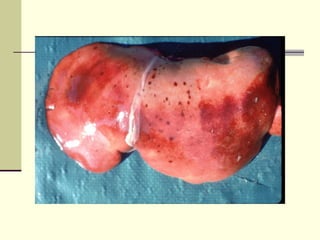
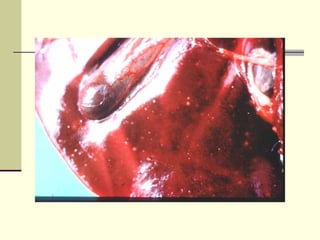
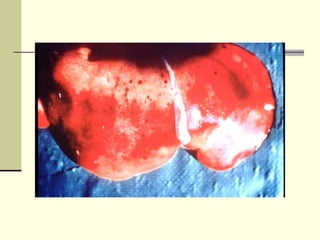
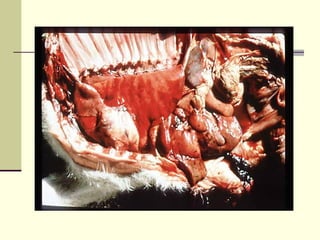
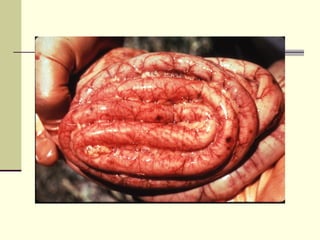
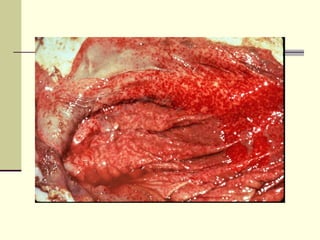
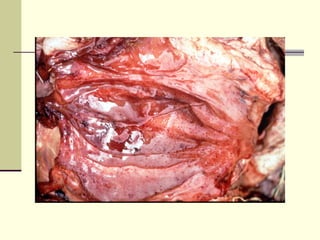
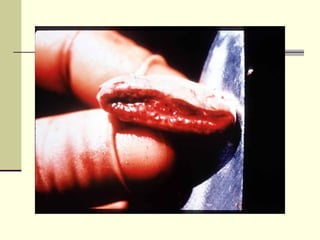
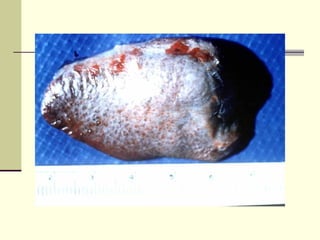
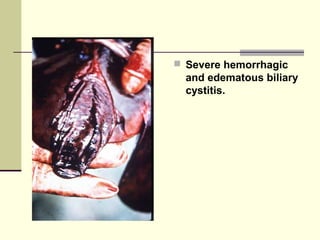
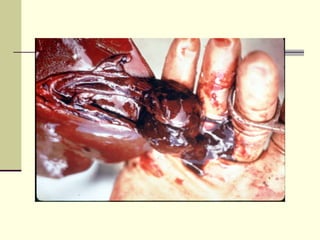
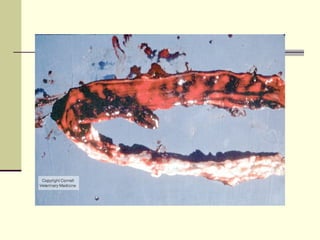
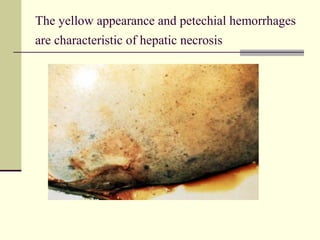
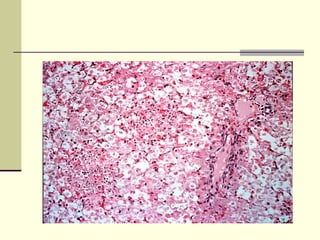
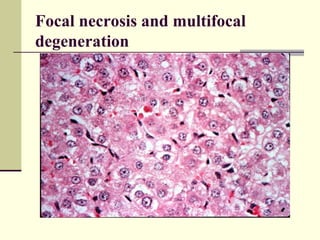
Rift Valley fever is an arthropod-borne viral disease that affects various mammals. It is characterized by abortions in pregnant animals and liver damage. The disease was first described in Kenya in 1931. It is endemic in many African and Middle Eastern countries. Transmission occurs via mosquito bites or contact with infected animal tissues. Symptoms in animals include fever, vomiting, and abortions. The virus can be diagnosed by isolating it from blood or tissues of infected hosts. Controlling mosquito populations and vaccinating susceptible animal species are important for prevention.