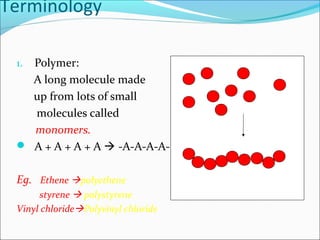
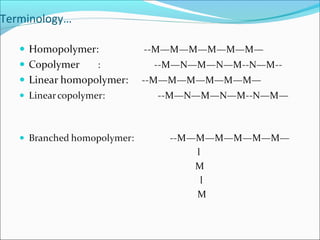
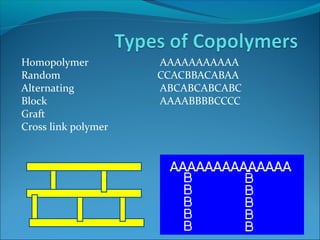
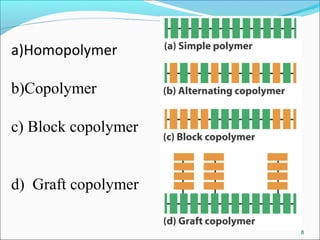
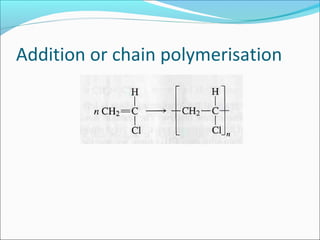
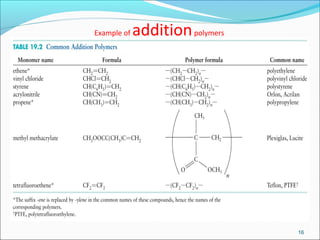
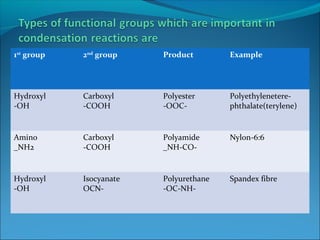
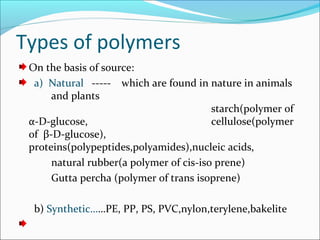
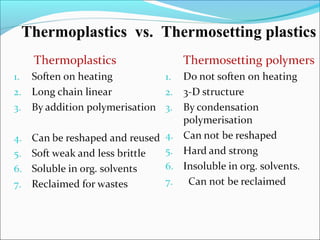
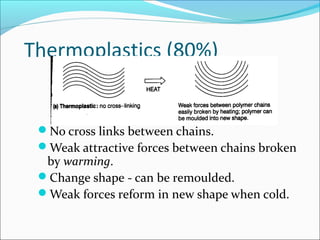
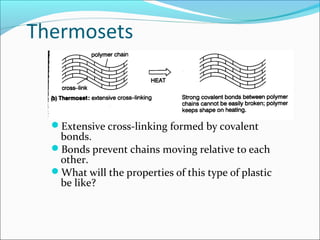
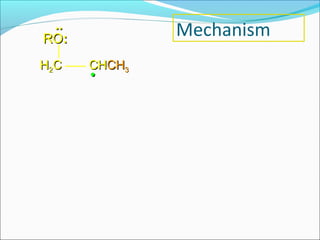
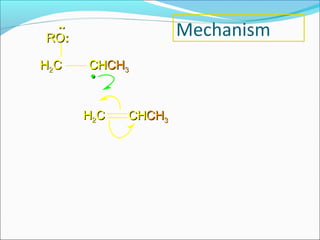
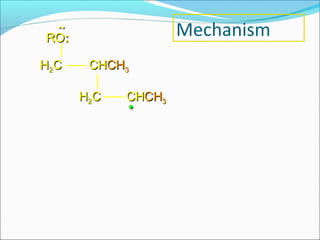
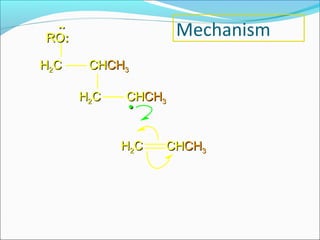
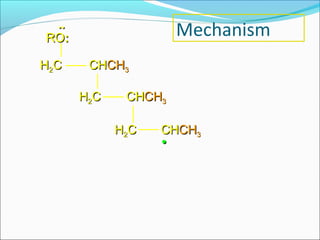
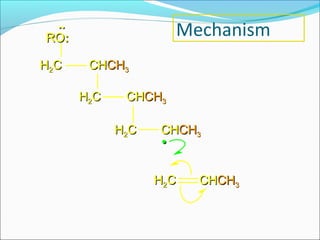
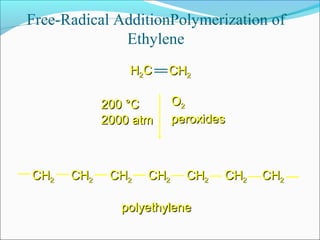
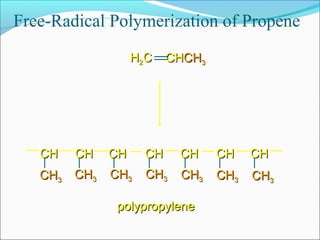
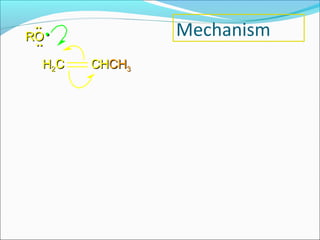
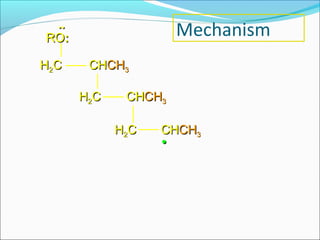
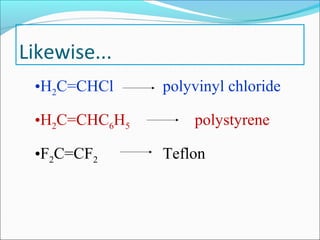
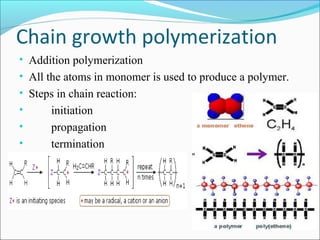
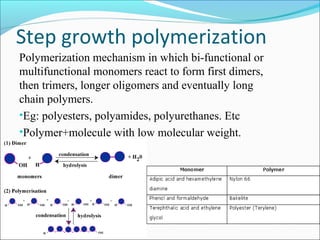
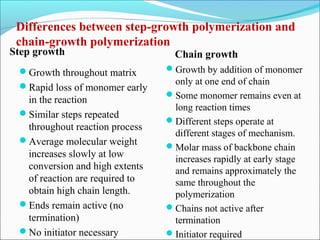
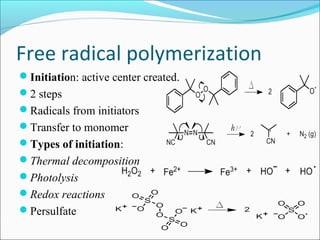
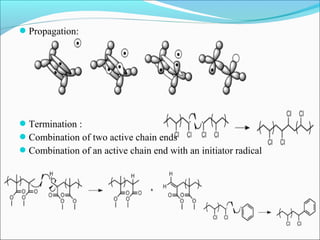
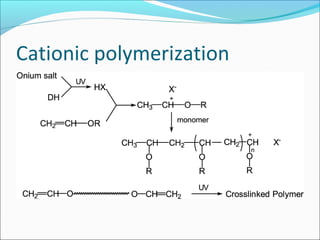
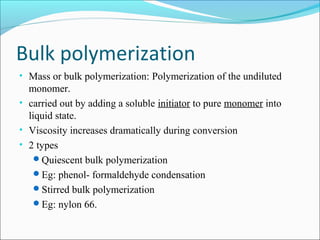
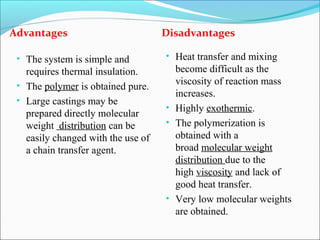
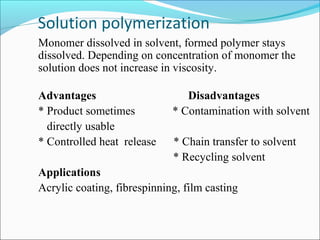
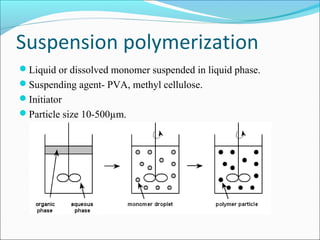
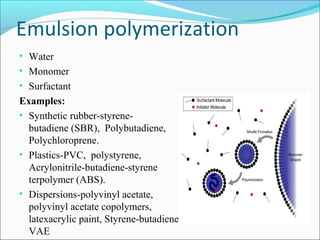
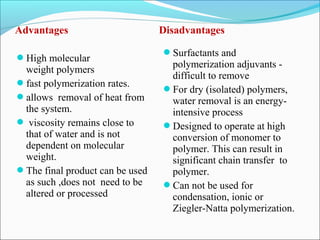
The document discusses various topics related to polymers including their classification, physical properties, types of polymerization, and important polymers. It describes the different types of polymers based on their source, structure, molecular forces, and provides examples. The key types of polymerization covered are addition, condensation, copolymerization, cationic and anionic polymerization. Important polymers discussed include polyethylene, polypropylene, polyvinyl chloride and their properties and uses.