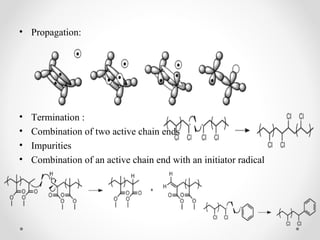
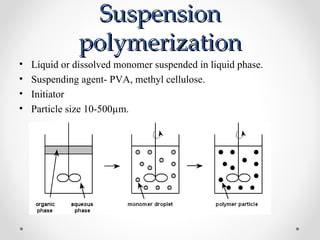
The document discusses various types of polymerization including chain growth polymerization methods like free radical, ionic, insertion and ring opening polymerization as well as step growth polymerization. It provides details on the mechanisms, monomers used, initiators, advantages and disadvantages of cationic, anionic, emulsion and other polymerization techniques.