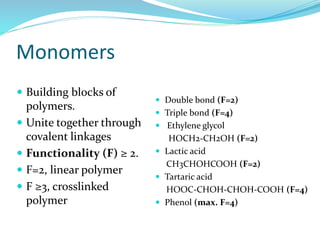
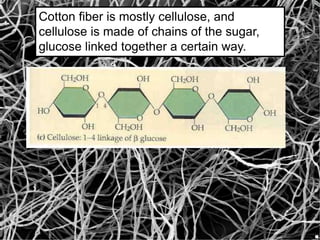
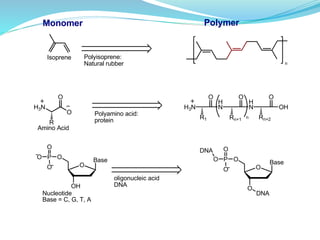
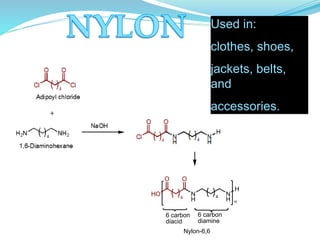
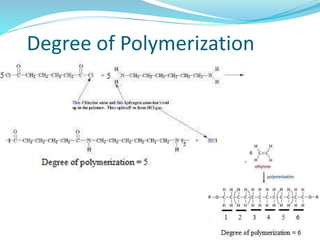
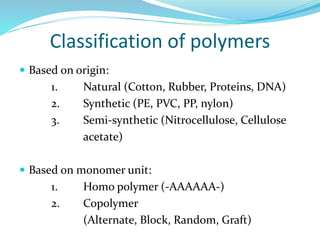
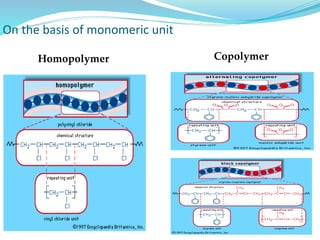
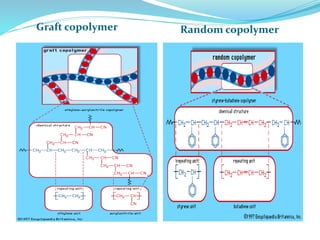
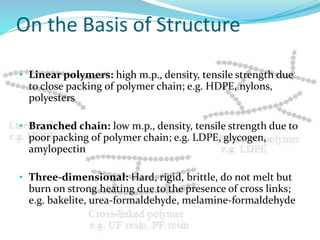
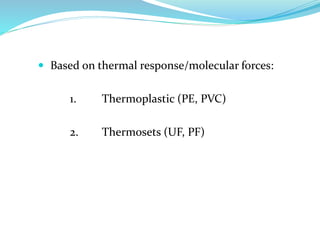
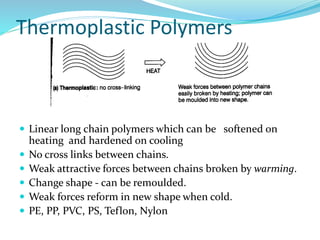
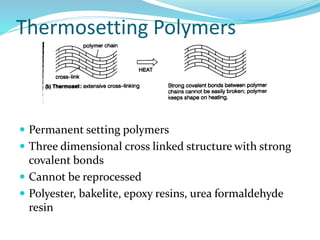
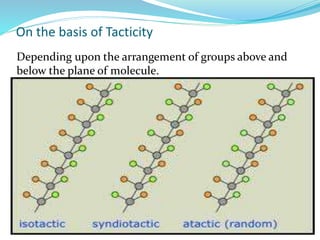
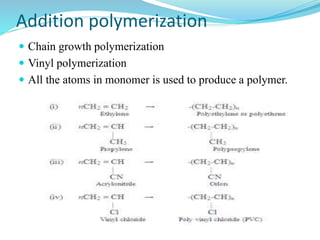
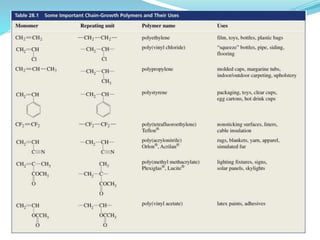
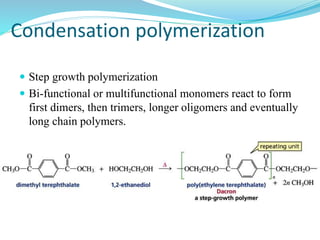
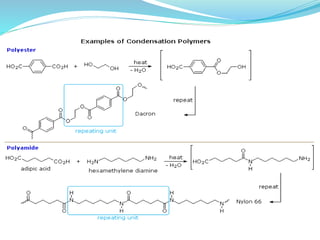
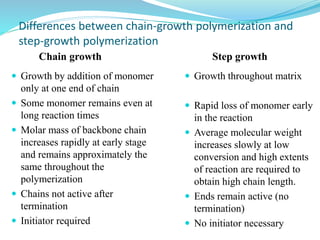
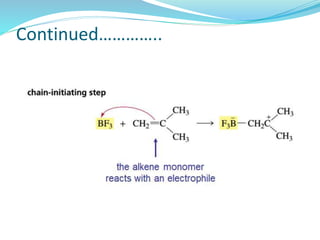
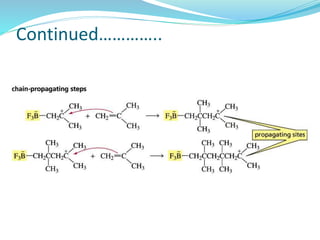
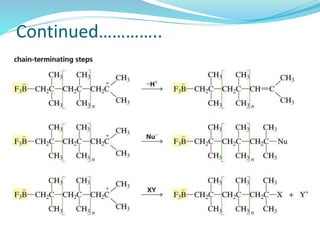
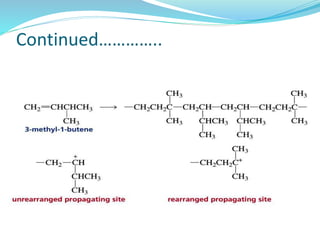
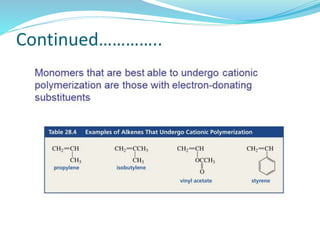
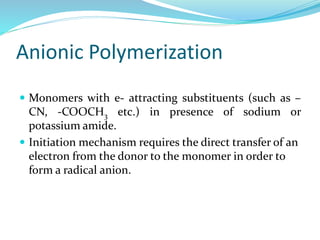
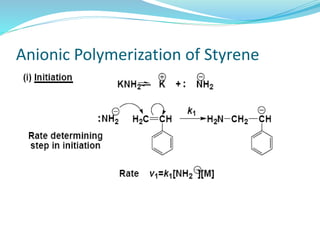
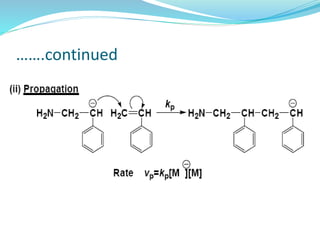
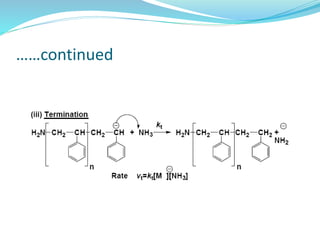
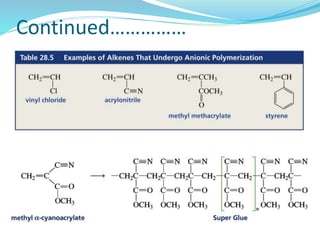
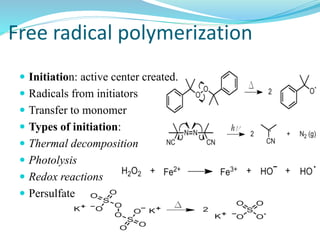
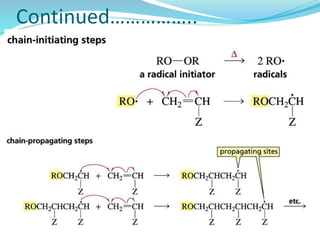
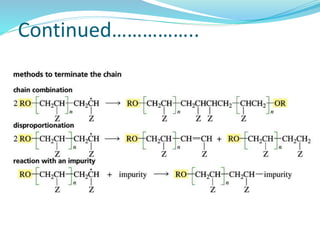
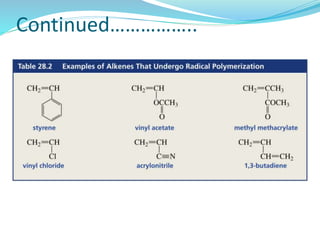
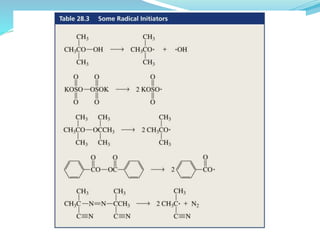
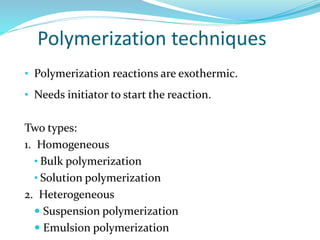
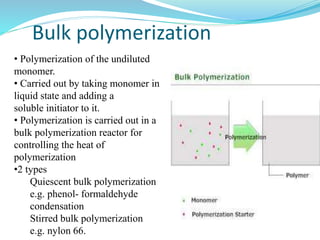
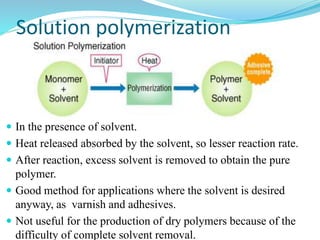
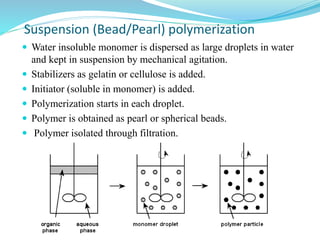
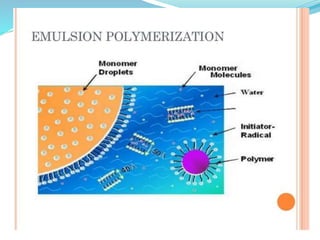
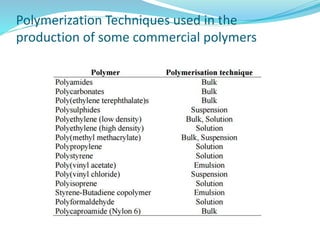
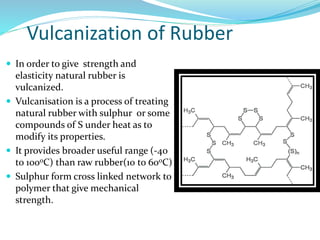
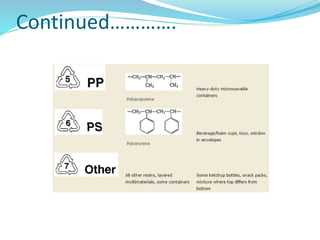
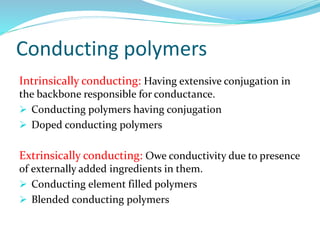
The document provides an overview of polymers, including their definition, properties, types, polymerization mechanisms, and applications. It categorizes polymers based on origin, structure, functionality, and response to heat, detailing processes such as addition and condensation polymerization. It also highlights advantages and uses of polymers, which include applications in everyday items like clothing, electrical appliances, and medical devices.