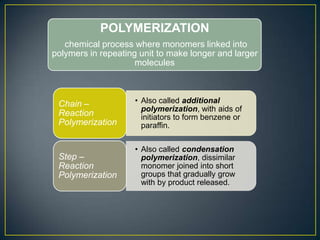
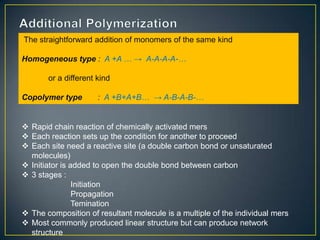
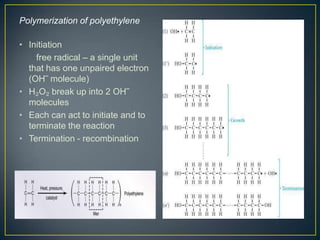
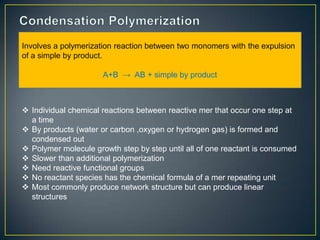
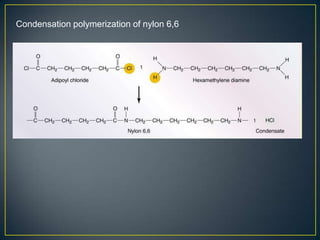
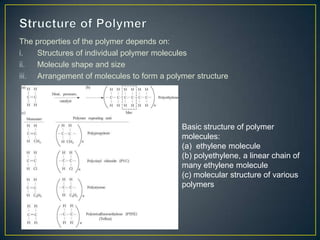
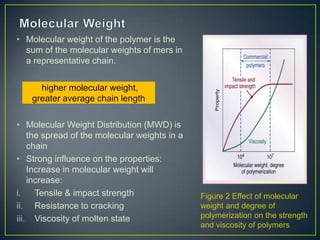
This document discusses natural and synthetic polymers. Natural polymers include collagen, gelatin, silk and wool. Synthetic polymers include polyethylene terephthalate, high density polyethylene, polyvinyl chloride, low density polyethylene and polypropylene. The document then discusses the structure of polymers including that they are made of repeating monomer units and can be formed through chain-reaction or step-reaction polymerization. It also discusses properties of polymers related to their molecular structure and weight.