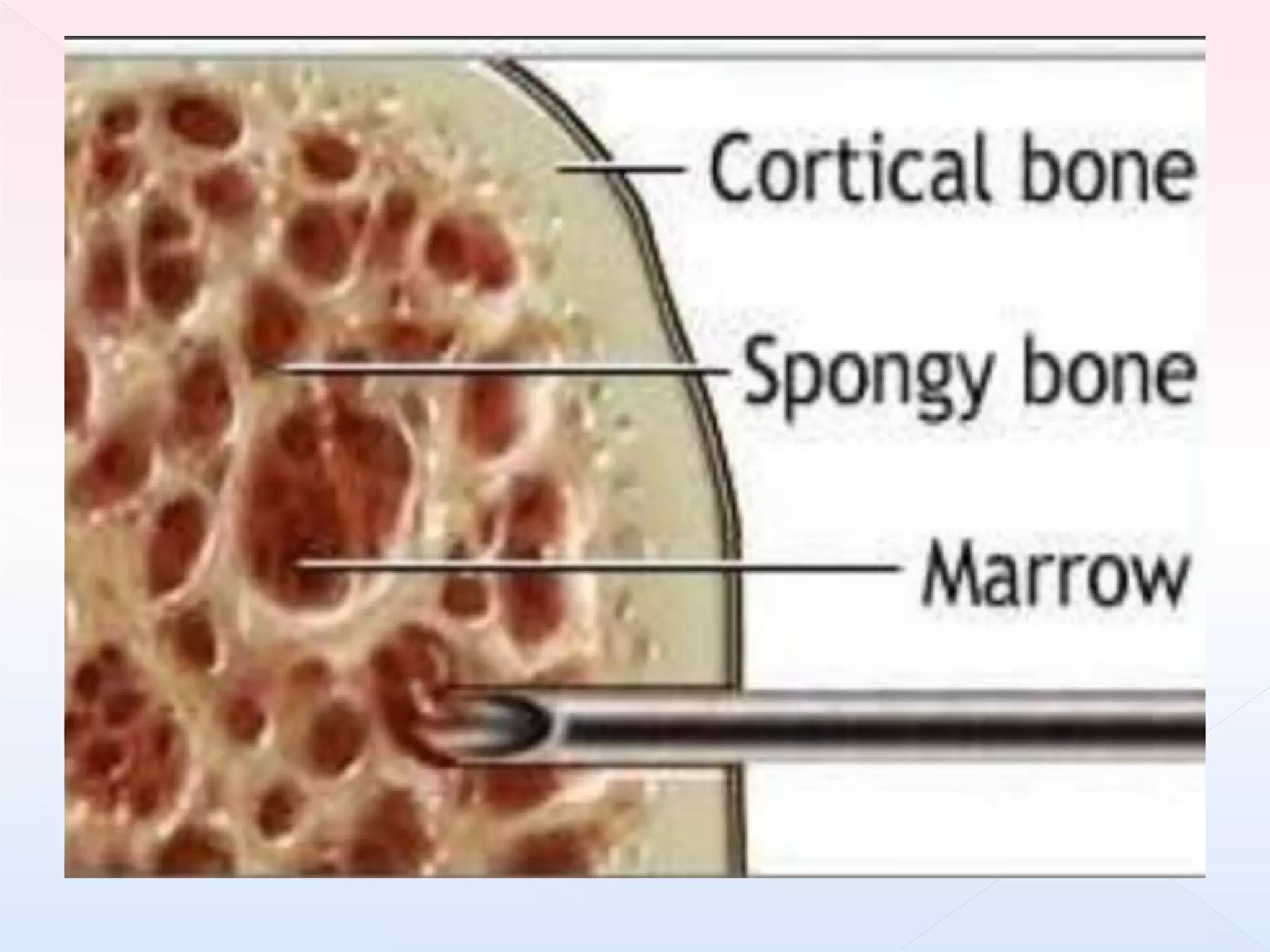
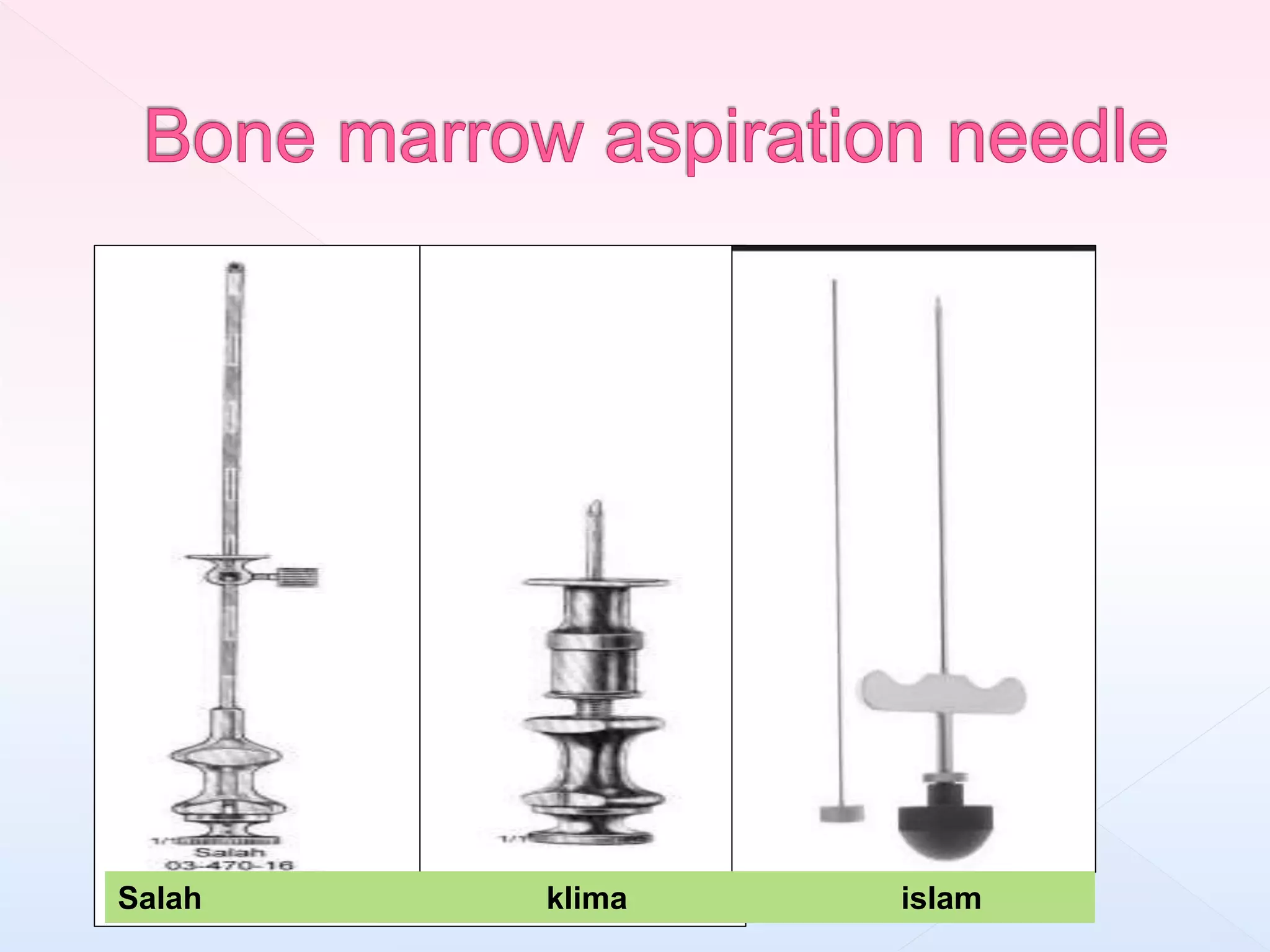
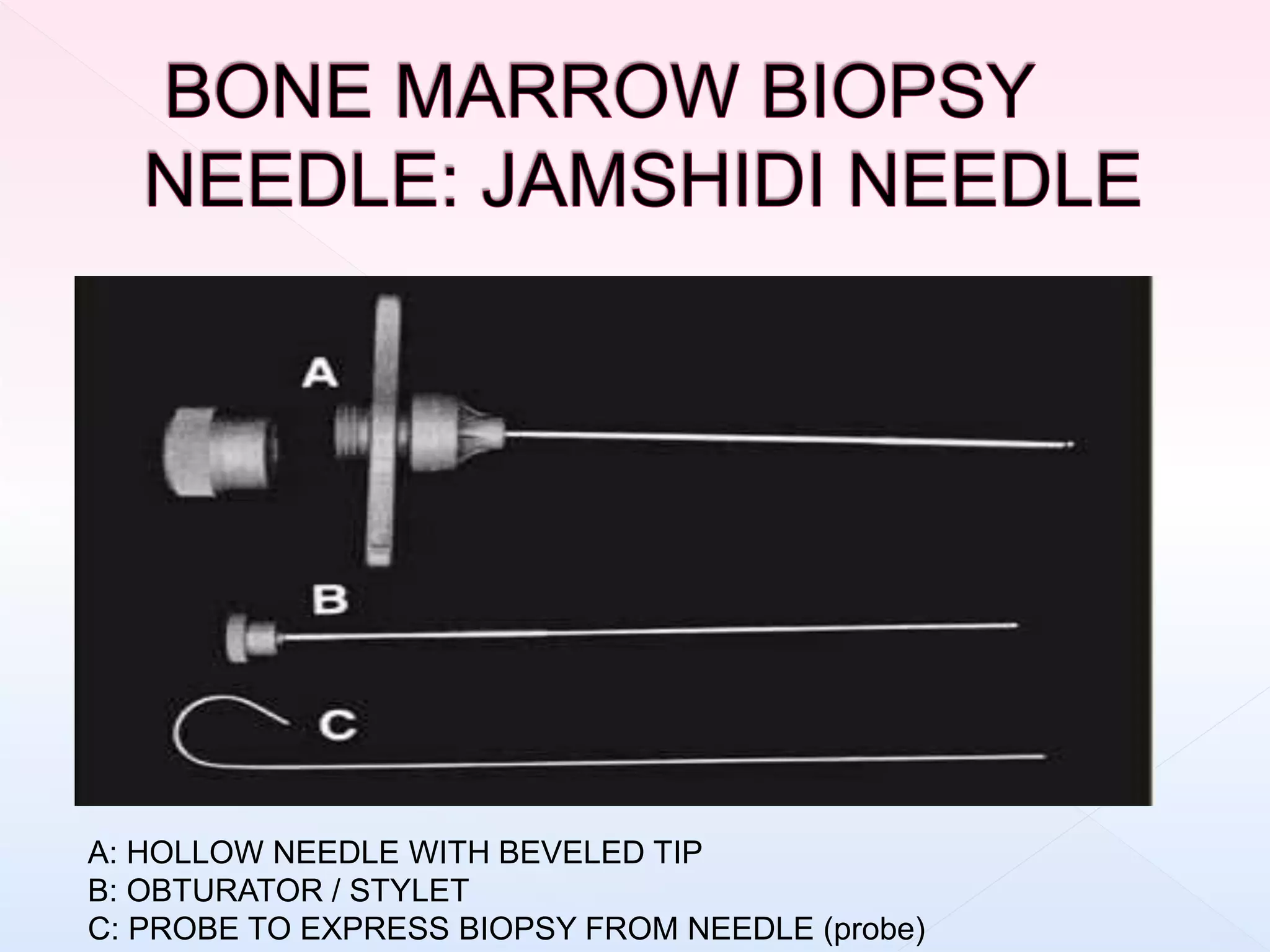
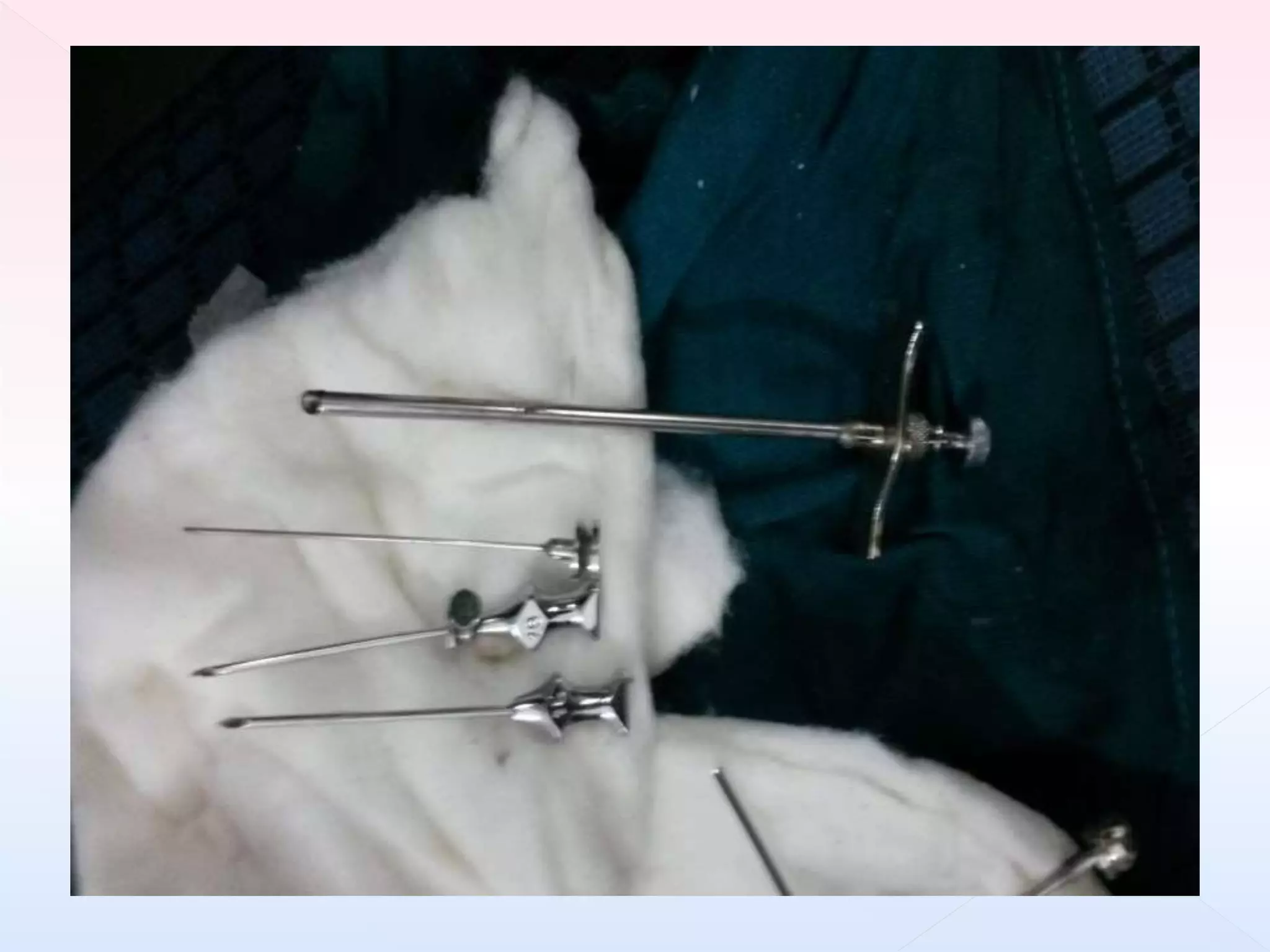
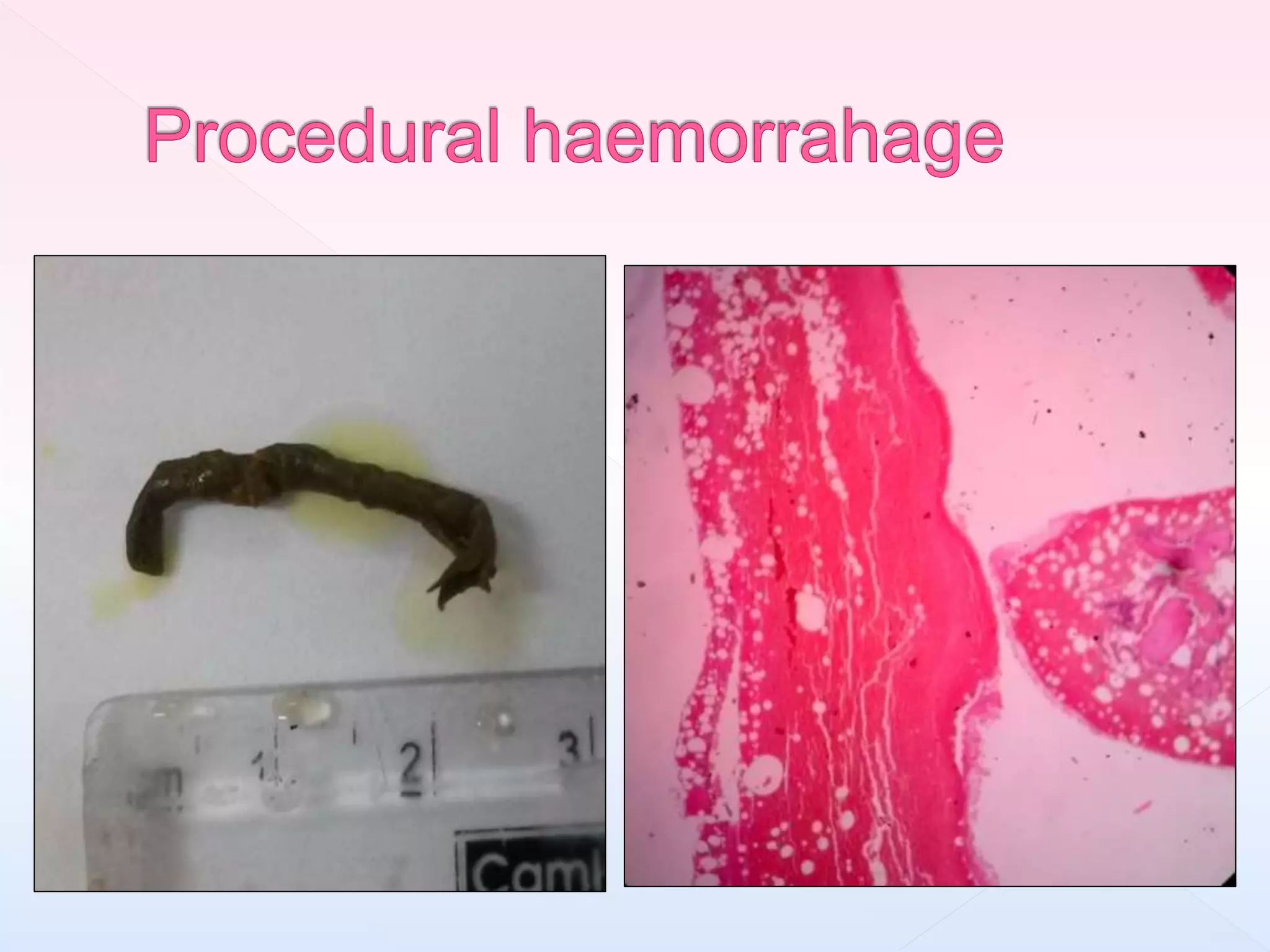
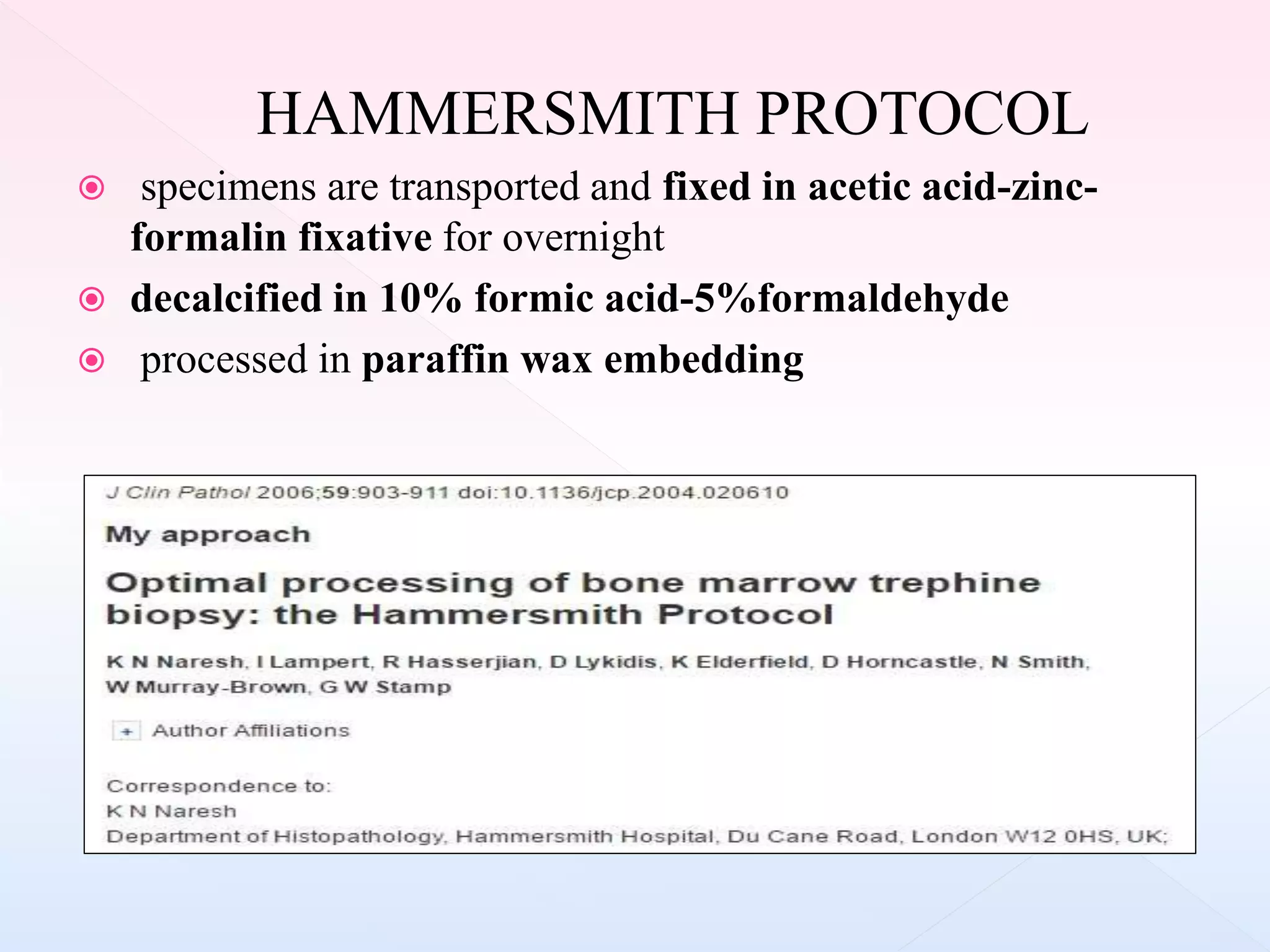
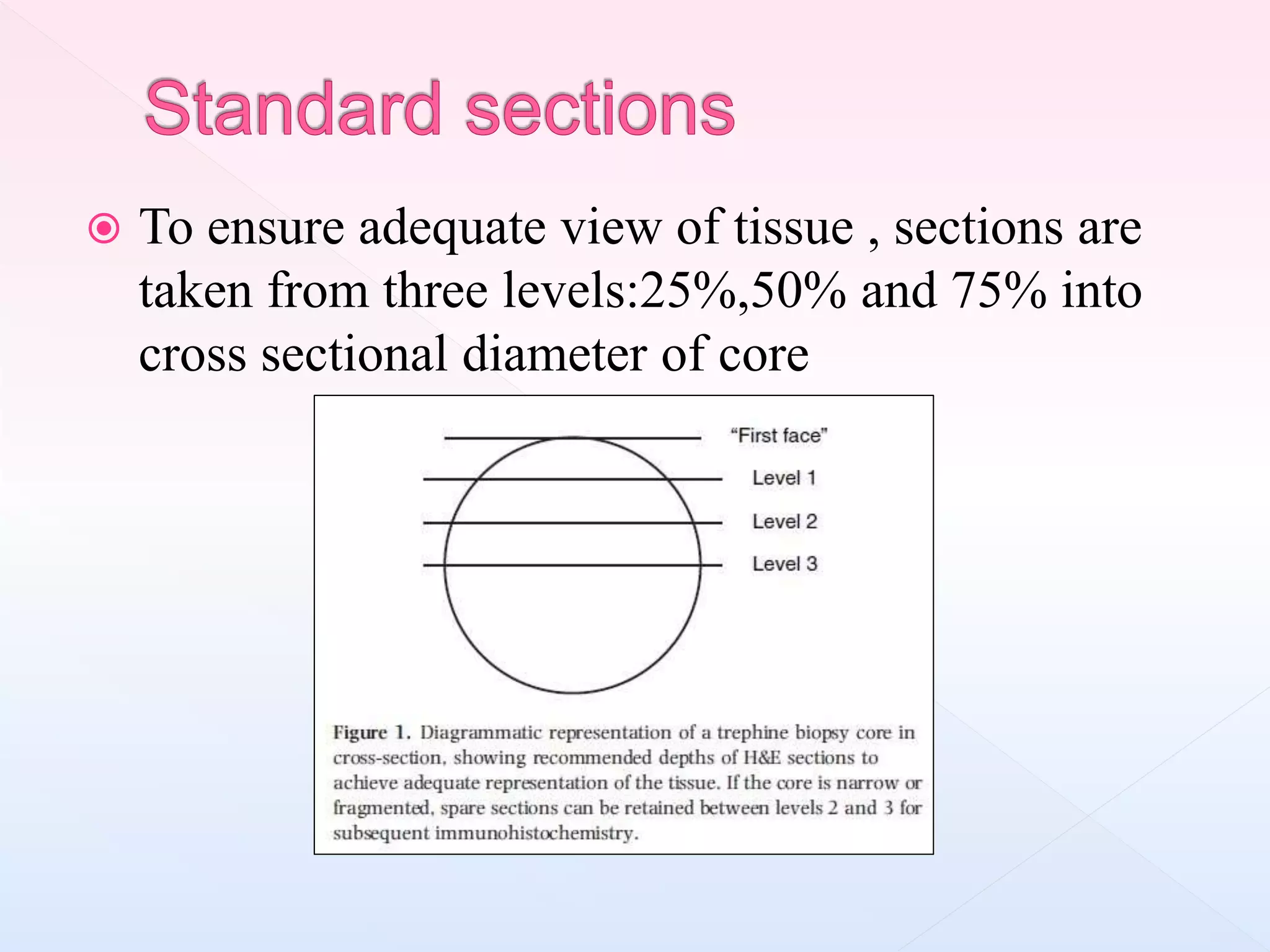
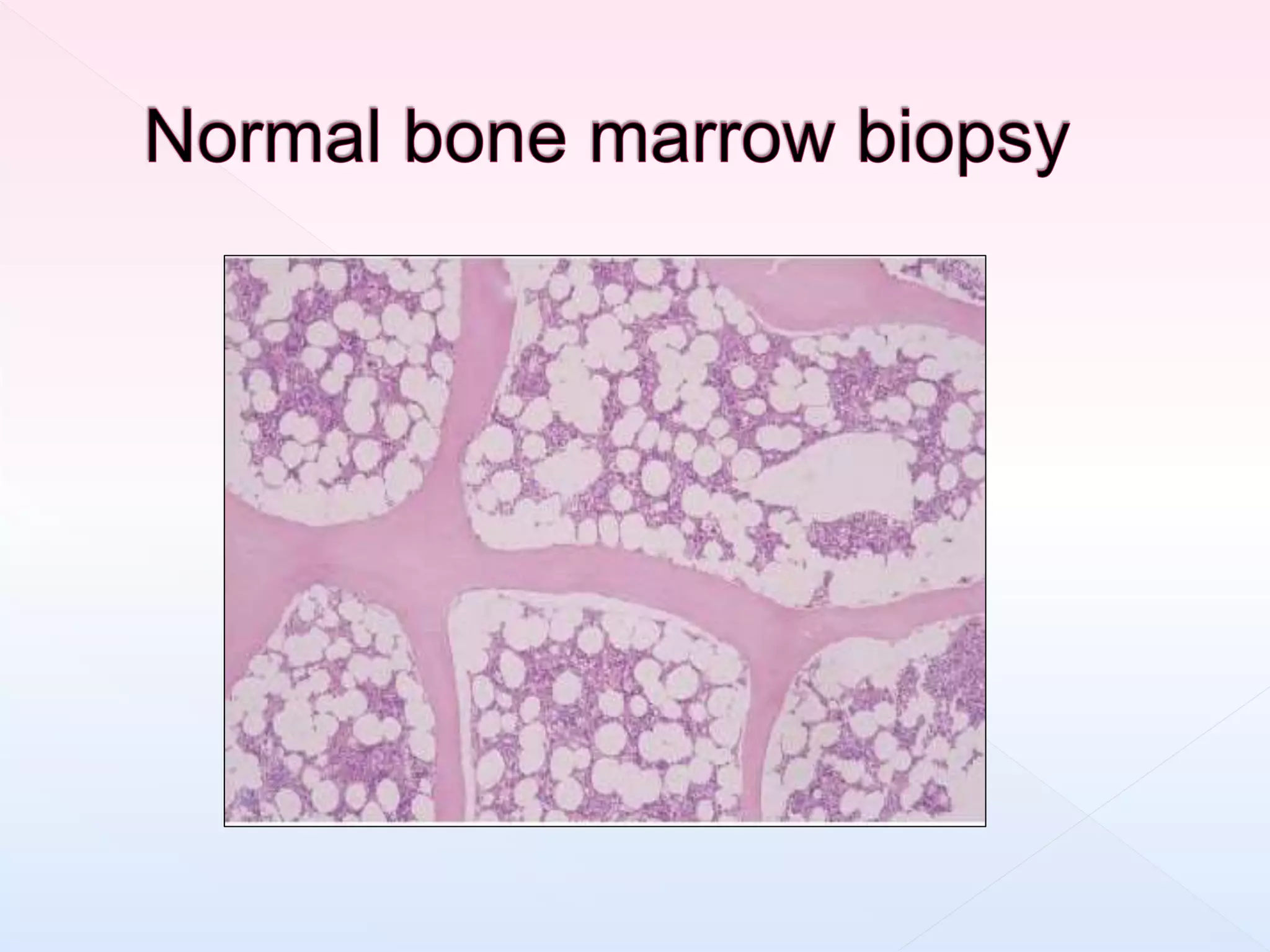
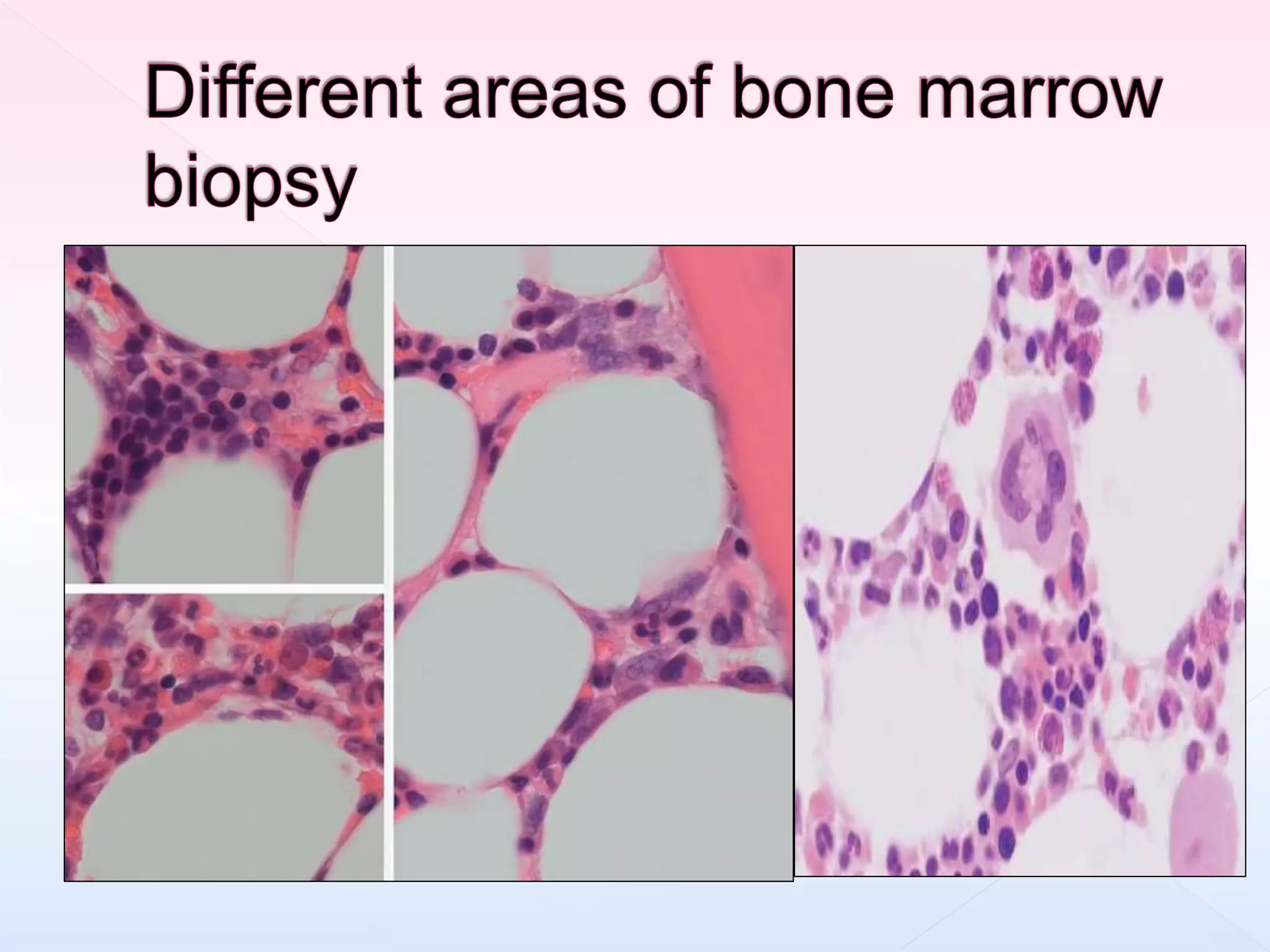
The document outlines procedures and considerations for bone marrow aspiration and biopsy, including indications for these procedures, contraindications, and details on specimen handling and fixation. It emphasizes the importance of proper decalcification techniques and the use of different fixatives to preserve morphology and molecular analysis capabilities. Additionally, it covers histological evaluations, staining methods, and the interpretation of cellularity and abnormal findings in bone marrow specimens.