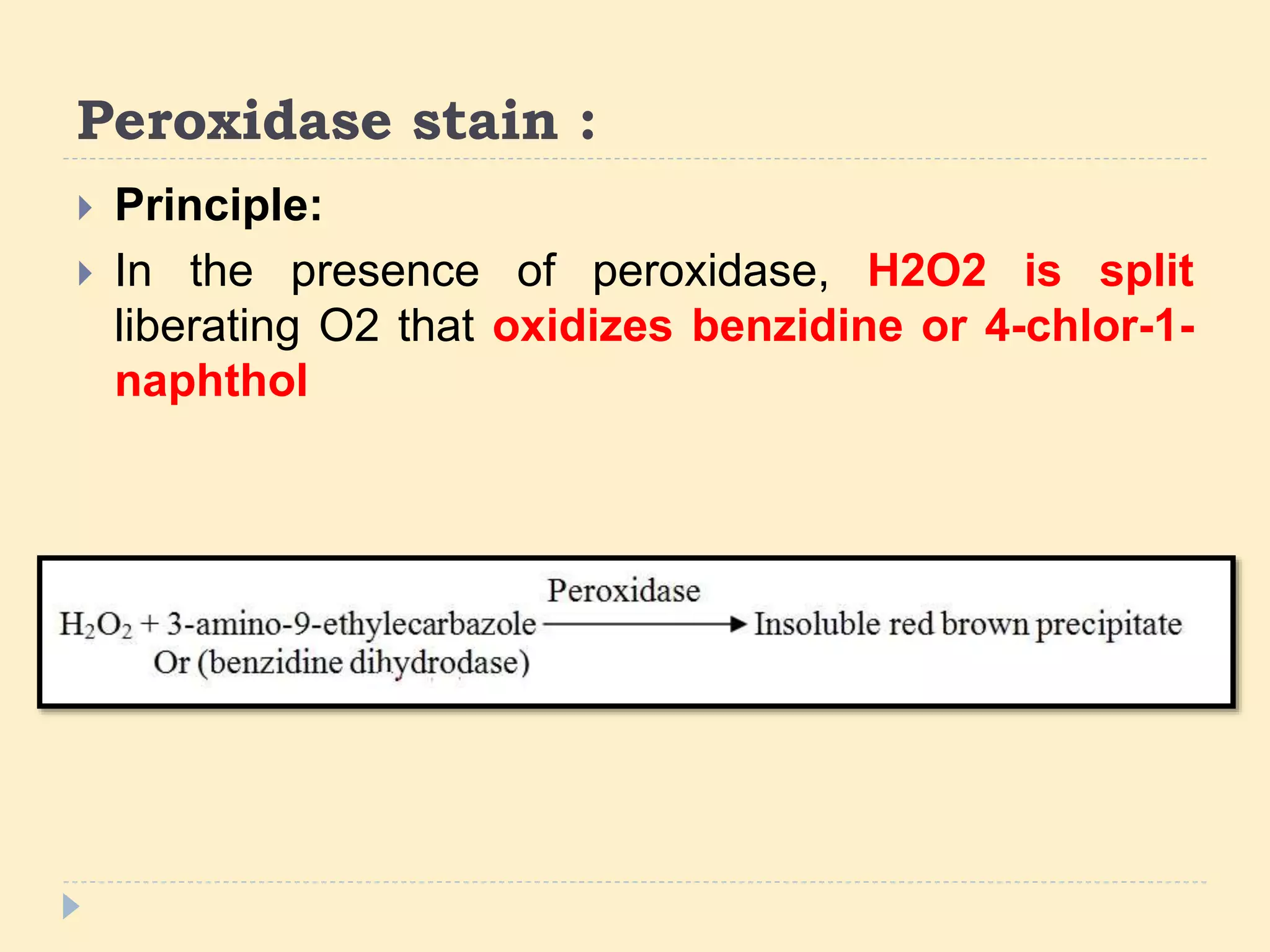
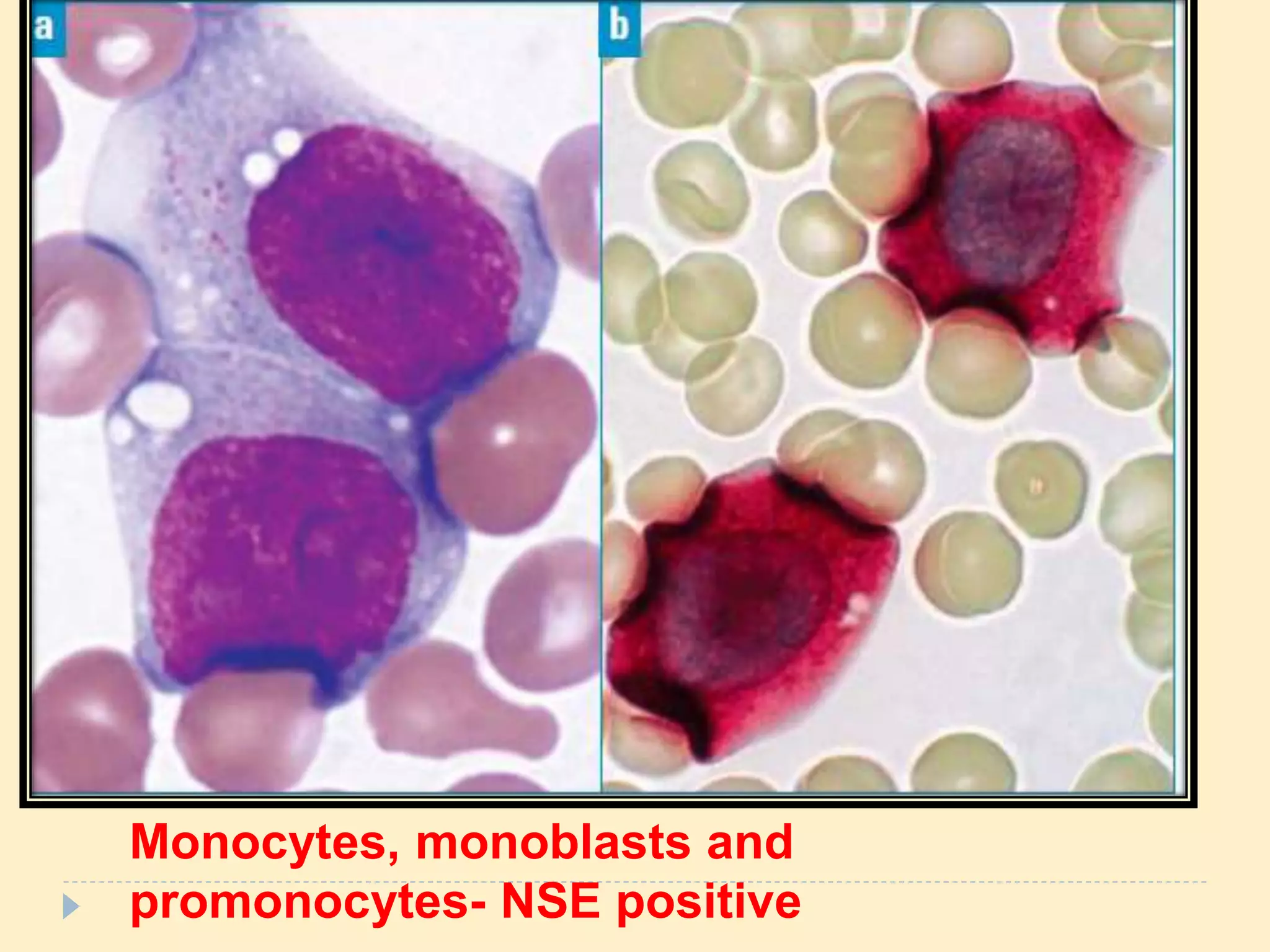
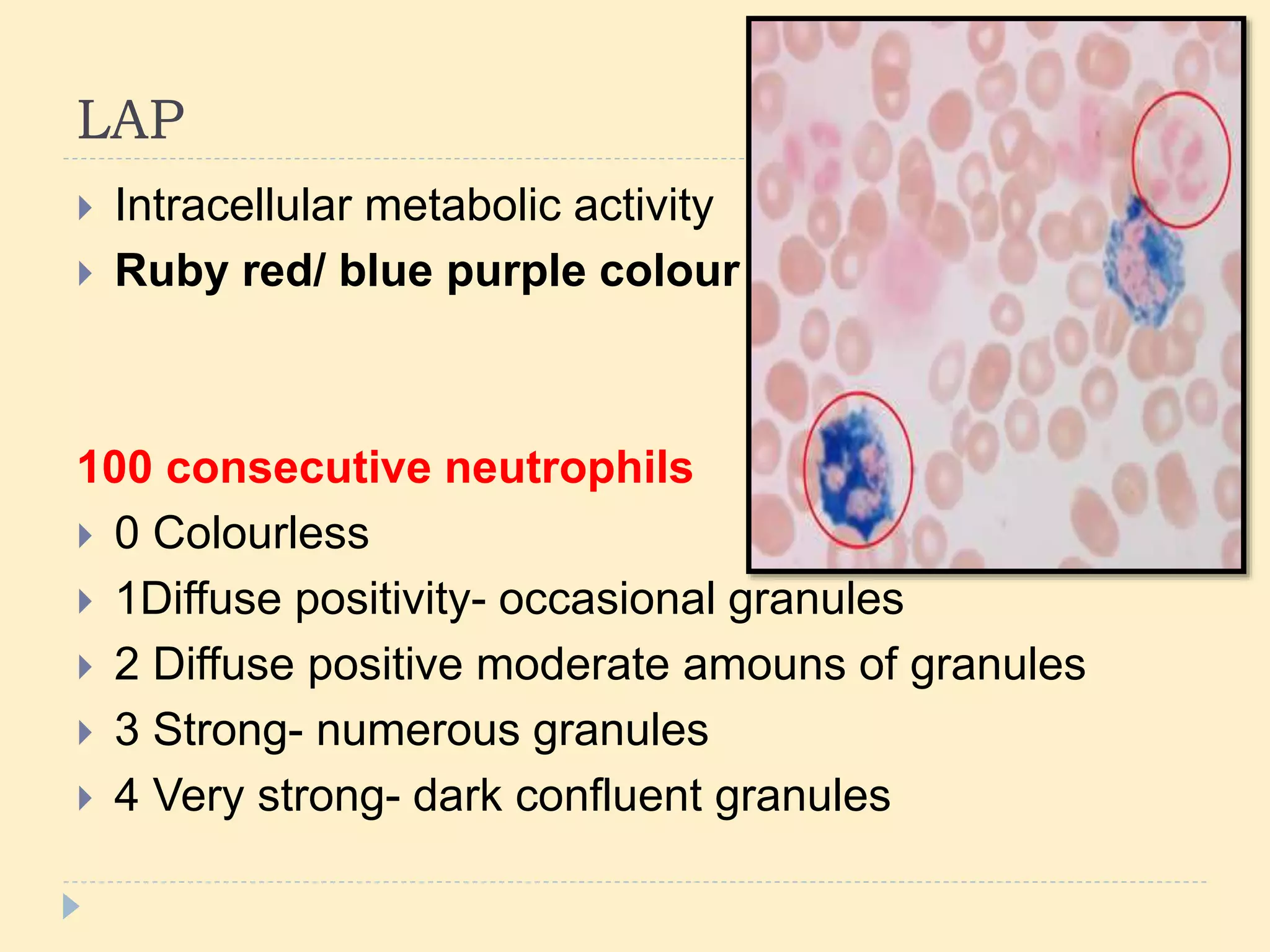
This document discusses special stains used in bone marrow examination, including Perls' Prussian blue stain for iron and Periodic acid–Schiff (PAS) stain. Perls' stain demonstrates iron stored as hemosiderin in macrophages and erythroblasts. The percentage of sideroblasts is assessed to evaluate iron availability. Ring sideroblasts are seen in certain myelodysplastic and myeloproliferative disorders. PAS stain demonstrates glycogen in granulocytes and megakaryocytes and can identify abnormal erythropoiesis. The staining patterns in acute leukemias are also described.


























![Periodic Acid – Schiff [PAS] Reaction
Periodic acid oxidizes the 1-2 glycol groups
in sugars, to produce dialdehydes
The oxidation condition has to be sufficiently
regulated so as to not oxidize the aldehydes further.
Aldehydes + Schiff reagent = purple-magenta
color.](https://image.slidesharecdn.com/newmicrosoftofficepowerpointpresentation-230921081028-9c4d2f51/75/Special-stains-in-Bone-marrow-examination-28-2048.jpg)