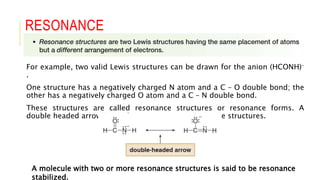
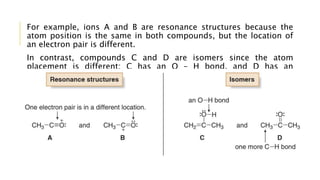
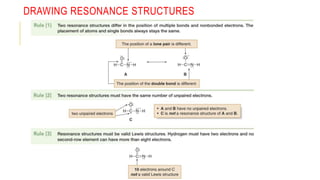
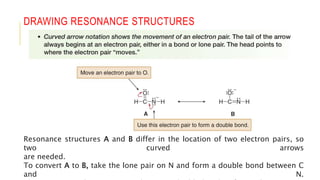
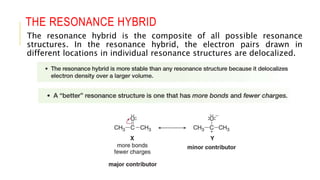
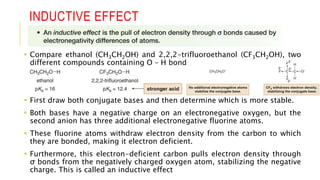
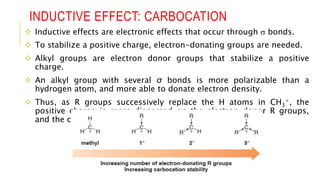
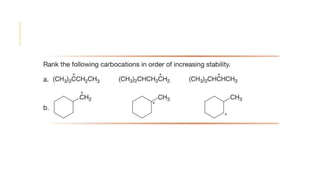
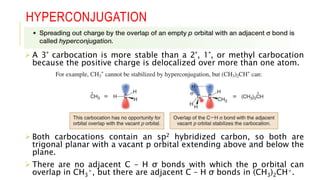
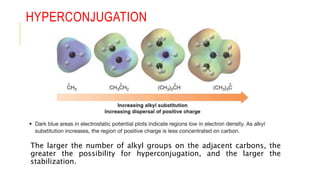
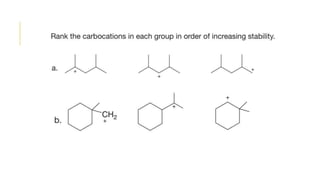
This document discusses inductive effects, resonance, and hyperconjugation. It defines resonance structures as alternative arrangements of electrons that stabilize molecules, and describes how resonance hybrids depict delocalized electrons. It explains that inductive effects stabilize charges through sigma bond polarization from electronegative atoms. Finally, it describes how hyperconjugation delocalizes positive charges in carbocations through overlap of vacant p orbitals with adjacent carbon-hydrogen sigma bonds.